Before explaining this 2006 study, it's better to start with the 2020 paper that first described the furin cleavage site unique to SARS-CoV-2:
https://t.co/1hLu6mIsuJ
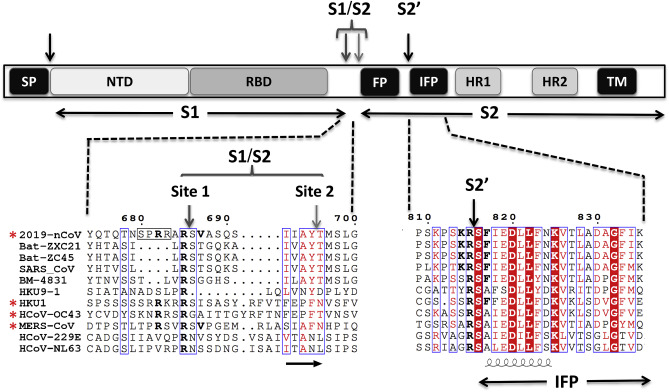
Coutard et al. observed SARS-CoV-2 "has a specific furin-like cleavage site absent in lineage b CoV including SARS-CoV sequences." Figure 2 is great because it aligns SARS-CoV-2 (aka 2019-nCoV) to not only SARS but also MERS and other CoVs with a known FCS marked with *.
Why is the FCS at the S1/S2 junction in the spike so important? A body of studies have shown that such a site can impact cell-to-cell fusion as well as the virus' ability to infect different cell types. This has been recently demonstrated in SARS-CoV-2 as well.
For example, in MERS-CoV, the S1/S2 FCS allows it to infect human lung and intestine (cancer derived) cells; without the FCS, the virus can only infect kidney (cancer derived) cells.
https://t.co/6hnaHsPlW0 and
https://t.co/rCP6DVFWbC
In SARS-CoV-2, when its FCS was lost during cell passage in monkey kidney cells, the virus became significantly attenuated in a hamster animal model of SARS2 disease.
https://t.co/qcxXkLeLaj
This was noticed by 2 groups who both commented that the FCS could be under strong selective pressure in humans or animals.
https://t.co/m8rfERr20t
Just to summarize before the next paper (1) S1/S2 FCS has not been seen in any beta CoVs, including all SARS-related CoVs, and (2) the S1/S2 FCS has been shown to confer pathogenic advantages even in SARS-CoV-2.
However, this paper reports a novel beta CoV, closely related to SARS-CoV-2, that has natural insertions (not demonstrated to be a functional FCS) at the S1/S2 junction.
https://t.co/Jy3lRn2K7C
Looking at their Fig 2 alignment of SARS-CoV-2 to the novel CoV RmYN02 and other SARS-related CoVs, others have pointed out that rather than an "insertion" maybe it is a unique alignment that results in a trimer shift, or maybe it's an insertion just downstream of a deletion?
Another way of aligning these sequences would have been to move that -NSPAA back to the left to fill that gap in the alignment. In that version, the novel CoV RmYN02 would look more similar to the other SARSr-CoVs - missing a 4 residue FCS in comparison to SARS-CoV-2.
But what Zhou et al. suggest is that this alignment of RmYN02 vs SARSr-CoVs "indicates that events of this kind are a natural and expected component of coronavirus evolution".
This is supported by their understanding that RmYN02 "is the closest relative of SARSCoV-2 in the long 1ab replicase gene, although the virus itself has a complex history of recombination".
After reading Zhou et al.'s paper I wanted to learn more about furin cleavage sites and coronaviruses so I simply searched for "furin" and "coronaviruses" on
https://t.co/lBViEQkdMN. One of the early papers was the 2006 study by Follis et al.
https://t.co/ngNdmyV8H2
Follis et al. were focusing on SARS-CoV but ultimately trying to understand why some viruses don't rely on an S1/S2 FCS to infect cells and mediate host cell-to-cell fusion.
Back in 2006, these were the other characterized CoVs (MERS FCS hadn't been studied yet) for comparison (Follis et al. Fig 1A). They noted: "the single arginine at position 667 of the SARS-CoV S glycoprotein, which may signal a protease-sensitive site".
To rule out that wild type (natural) SARS-CoV wasn't already getting cleaved at this site without a notable FCS, Follis et al. mutated that arginine at position 667 (aka R667) and found no impact on Spike expression or glycosylation, confirming no evidence of cleavage.
Then, Follis et al. engineered the BCoV RRSR(R677) FCS into SARS to see what would happen (Fig 1B). They found that for cleavage to occur, you had to insert an extra residue similar to the BCoV alignment, and not perturb the sequence downstream of R677.
"cleavage of the SARS S glycoprotein can be achieved by the introduction of a furin recognition motif, especially at R667. The differences in the degree of cleavage between the two R667 mutants, and in SLLR, suggest specificity in the recognition of the engineered furin sites"
At this point, I want to draw your attention back to Coutard et al.'s figure which shows an updated list of CoVs and their FCS sites: SARS2 SPRRAR, HKU1 SRRKRR, OC43 KNRRSR, MERS TPRSVR. In many naturally found CoVs, this FCS at R667 exists and the sequence can be quite flexible.
Follis et al. showed improved cell-to-cell fusion by the SARS Spike with the inserted FCS. However, they only tested the infectivity of Spike-presenting particles on human 293T kidney cells. We know now the FCS does not affect kidney cell infection, but impacts other cell types.
To summarize, the S1/S2 FCS exists in natural CoVs, but has not been observed in SARS-related-CoVs. Scientists tested introduction of FCS at R667 in SARS. Later discovered in MERS that FCS enables infection of more cell types. Now we know SARS2 FCS confers infection advantages.
Ending of another unsatisfying tweetorial: there is zero evidence that confirms that the SARS-CoV-2 S1/S2 PRRA(R) FCS arose naturally or artificially, but neither scenario can be ruled out.
A later paper by a different group, ending with the suggestion to alter the FCS to see if that influences host range or viral pathogenesis.
https://t.co/EvLJ1Gn81s
Intriguing observation by others that the S1/S2 FCS in SARS-CoV-2 may act also as a heparan sulfate binding site, which may extend the host range of the virus.
https://t.co/uDgl5nct1G
A mouse CoV that was passaged in vitro to expand its host range was found to have acquired 2 new HS binding sites while preserving the one in the S1/S2 FCS.
https://t.co/IeIR4BI9Sl
Which is why Belouzard et al. said that it was possible that intro'ing S1/S2 FCS in SARS "serendipitously created a basic heparin sulfate-binding site, and so we cannot rule out the possibility of cross-talk between heparin sulfate-binding and furin cleavage for SARS-CoV S".
Although de Haan et al. found that the mouse CoV with the extended host range actually lost the ability to be cleaved at the S1/S2 FCS, there is preliminary research to suggest furin inhibitors may be useful against SARS2, possibly due to FCSs other than the one at the S1/S2 jxn.
"Our data demonstrate that both TMPRSS2 and furin are essential for SARS-CoV-2 activation in human airway cells and are promising drug targets for the treatment of COVID-19"
https://t.co/SUGDvwUnSO
I started looking into this because of reports that heparin (a commonly used blood thinner in hospitals) can help in severe cases of COVID-19 with deep vein blood clotting.
https://t.co/VVwc6idxg8
But heparin treatment could go beyond blood clotting and may play a role in blocking virus invasion of cells:
https://t.co/lPTQOo1TPj
Discovery (via Twitter) of a 2017 paper by Shi Zhengli and YunZhi Zhang describing an S1/S2 RRAR potential cleavage site in a rat alpha CoV. But it's not at the same R in the spike.
https://t.co/78mJdVDJZe