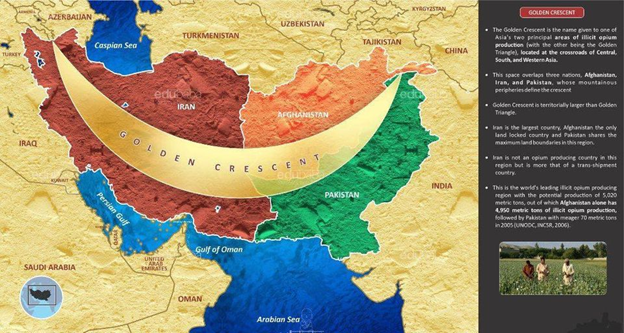
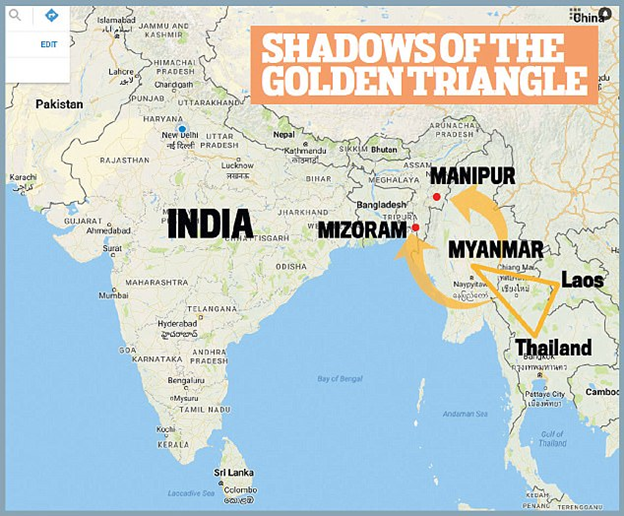
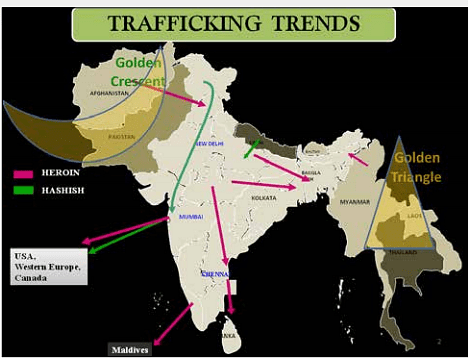
What does this have to do with steel?
Did you know that all the nuclear bombs we exploded in the '40s-50s-60s have permanently contaminated all the steel the world has produced since then? All steel in the world is divided into two categories: pre-1945 non-contaminated steel, and post-1945 contaminated steel.
How?
What does this have to do with steel?
So we need pre-1945 steel
We go, find pre-World-War-II sunken shipwrecks, and then salvage the steel (and lead) from those to build our instruments which need heavy radiation shielding.
Apparently, this Cesium-137 test is used in practice to detect wine and art forgery https://t.co/51nqVfWb7W
— Shreyas Panhalkar (@achillesHeelV2) March 9, 2021
https://t.co/nzmZGSKPe3
Does that mean the entire population of earth which is breathing this air for atmospheric oxygen is also inhaling Conalt-60?
— Pradeep Hardikar (@PradeepHardikar) March 9, 2021
More from Science
💥and so it begins..💥
It's time, my friends 🤩🤩
[Thread] #ProjectOdin
https://t.co/fO90N78fta
new quantum-based internet #ElonMusk #QVS #QFS
Political justification ⏬⏬
#ProjectOdin
#ProjectOdin #Starlink #ElonMusk #QuantumInternet
It's time, my friends 🤩🤩
[Thread] #ProjectOdin
The Alliance has Project Odin ready to go - the new quantum-based internet. #ElonMusk #QVS #QFS #ProjectOdin
— Der Preu\xdfe Parler: @DerPreusse (@DerPreusse1963) January 12, 2021
https://t.co/fO90N78fta

new quantum-based internet #ElonMusk #QVS #QFS
Political justification ⏬⏬
#ProjectOdin

#ProjectOdin #Starlink #ElonMusk #QuantumInternet

What are the classics of the "Science of Science" or "Meta Science"? If you were teaching a class on the subject, what would go in the syllabus?
Here's a (very disorganized and incomplete) handful of suggestions, which I may add to. Suggestions welcome, especially if you've dug into relevant literatures.
1. The already classic "Estimating the reproducibility of
psychological science" from the Open Science Collaboration of @BrianNosek et al. https://t.co/yjGczLZ6Je
(Look at that abstract, wow!)
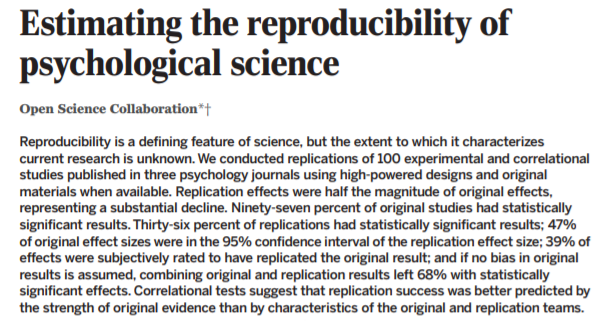
Many people had pointed out problems with standard statistical methods, going back decades (what are the best refs?). But this paper was a sledgehammer, making it impossible to ignore the question: what, if anything, were we actually learning from all those statistical studies?
2. Dean Keith Simonton's book "Creativity in Science: Chance, Logic, Genius, and Zeitgeist". If an essentially scientometric book could be described as a fun romp through science & creativity, this would be it
Here's a (very disorganized and incomplete) handful of suggestions, which I may add to. Suggestions welcome, especially if you've dug into relevant literatures.
1. The already classic "Estimating the reproducibility of
psychological science" from the Open Science Collaboration of @BrianNosek et al. https://t.co/yjGczLZ6Je
(Look at that abstract, wow!)

Many people had pointed out problems with standard statistical methods, going back decades (what are the best refs?). But this paper was a sledgehammer, making it impossible to ignore the question: what, if anything, were we actually learning from all those statistical studies?
2. Dean Keith Simonton's book "Creativity in Science: Chance, Logic, Genius, and Zeitgeist". If an essentially scientometric book could be described as a fun romp through science & creativity, this would be it
You May Also Like
“We don’t negotiate salaries” is a negotiation tactic.
Always. No, your company is not an exception.
A tactic I don’t appreciate at all because of how unfairly it penalizes low-leverage, junior employees, and those loyal enough not to question it, but that’s negotiation for you after all. Weaponized information asymmetry.
Listen to Aditya
And by the way, you should never be worried that an offer would be withdrawn if you politely negotiate.
I have seen this happen *extremely* rarely, mostly to women, and anyway is a giant red flag. It suggests you probably didn’t want to work there.
You wish there was no negotiating so it would all be more fair? I feel you, but it’s not happening.
Instead, negotiate hard, use your privilege, and then go and share numbers with your underrepresented and underpaid colleagues. […]
Always. No, your company is not an exception.
A tactic I don’t appreciate at all because of how unfairly it penalizes low-leverage, junior employees, and those loyal enough not to question it, but that’s negotiation for you after all. Weaponized information asymmetry.
Listen to Aditya
"we don't negotiate salaries" really means "we'd prefer to negotiate massive signing bonuses and equity grants, but we'll negotiate salary if you REALLY insist" https://t.co/80k7nWAMoK
— Aditya Mukerjee, the Otterrific \U0001f3f3\ufe0f\u200d\U0001f308 (@chimeracoder) December 4, 2018
And by the way, you should never be worried that an offer would be withdrawn if you politely negotiate.
I have seen this happen *extremely* rarely, mostly to women, and anyway is a giant red flag. It suggests you probably didn’t want to work there.
You wish there was no negotiating so it would all be more fair? I feel you, but it’s not happening.
Instead, negotiate hard, use your privilege, and then go and share numbers with your underrepresented and underpaid colleagues. […]