What are CROs, CDMOs, CMOs and CRAMS? How are they different from each other and what value do they add to the Pharma Value Chain? To understand these concepts, we took a look at the drug discovery process.
Thread on Drug Development process 🧵
Hit like & Retweet for more learnings ! 💊🧪🩺💉
@unseenvalue
Topics Covered :
1.Introduction to CRO,CDMO,CMO & CRAMS
2.Discovery
3.Clinical Trials
4.Commercialization and Surveillance
5.Success Rates
6.CRO
7.CMO
8.CDMO
9.CRAMS
What are CROs, CDMOs, CMOs and CRAMS? How are they different from each other and what value do they add to the Pharma Value Chain? To understand these concepts, we took a look at the drug discovery process.
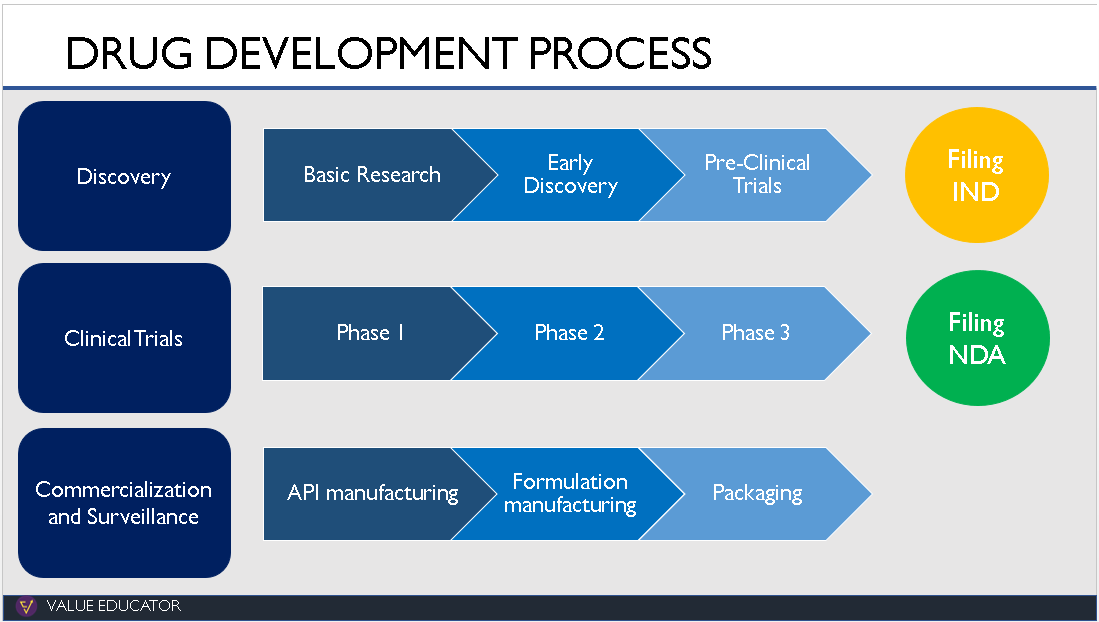
The discovery of a new drug starts in the research lab where researchers study hundreds of diseases. They analyse what part of the body a disease affects and what reaction the body has to these diseases.
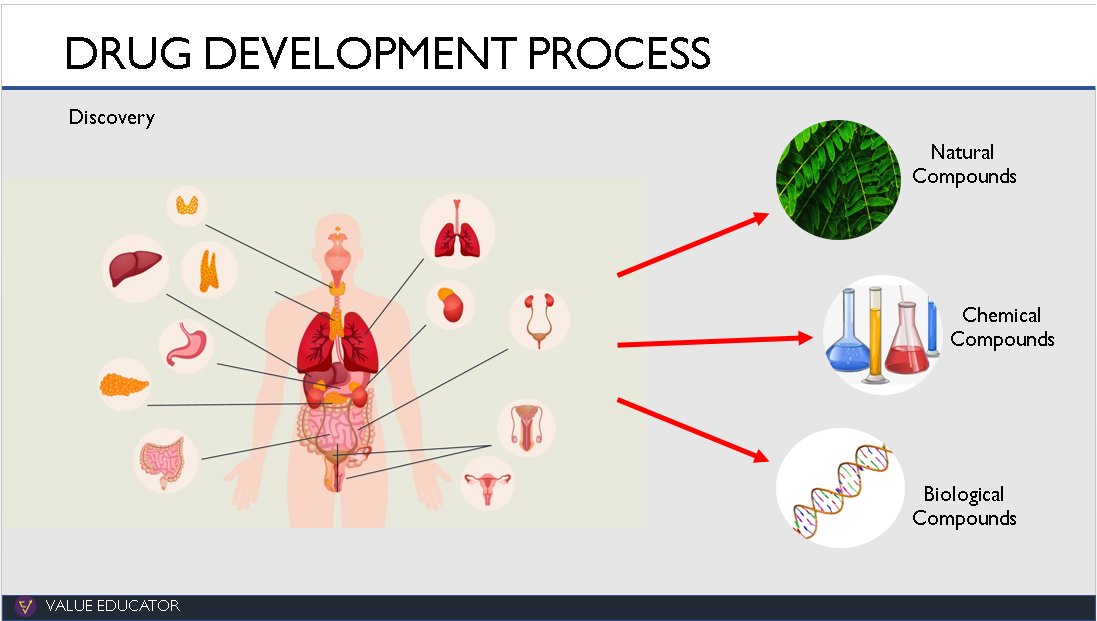
Once all this data has been collected, they file an Investigational New Drug (IND) Application with the US FDA. The US FDA reviews the IND within 6 months of filing. Once the IND is approved,
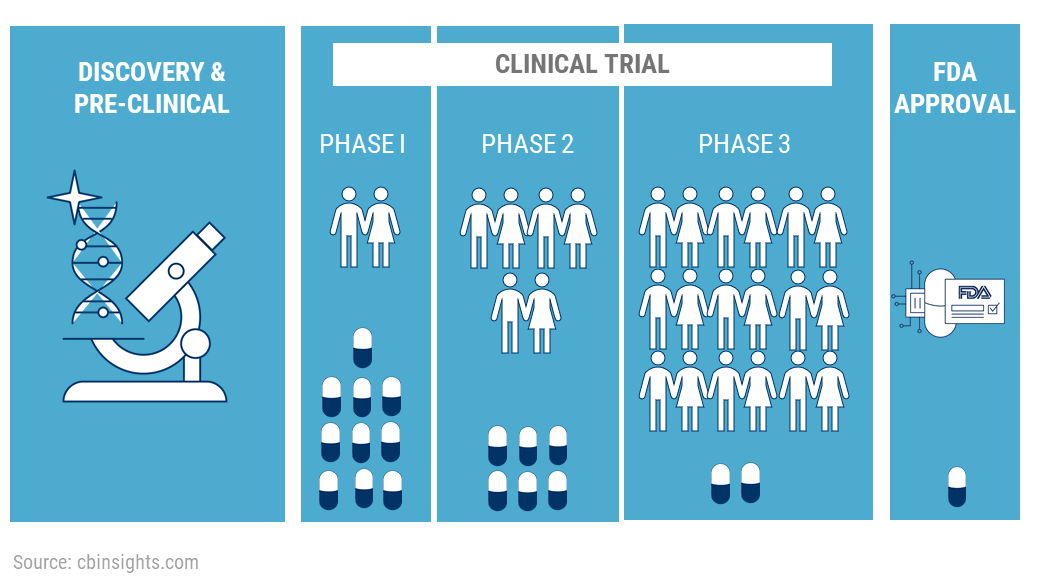
3. Clinical Trials:
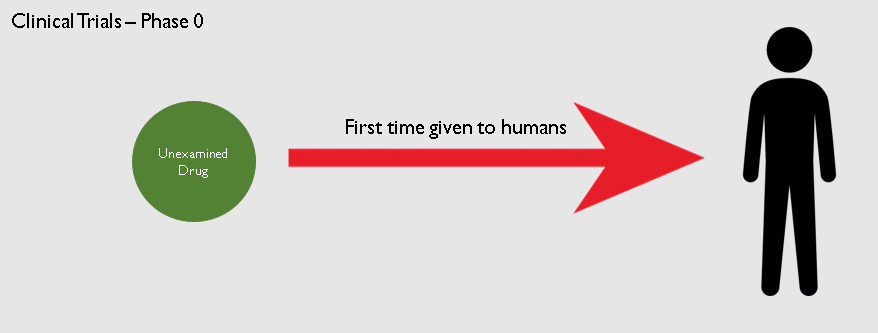
The drug now moves to the Clinical Trials phase. It will be the first time a novel drug will be given to human beings. The clinical trials itself take place in 3 phases.
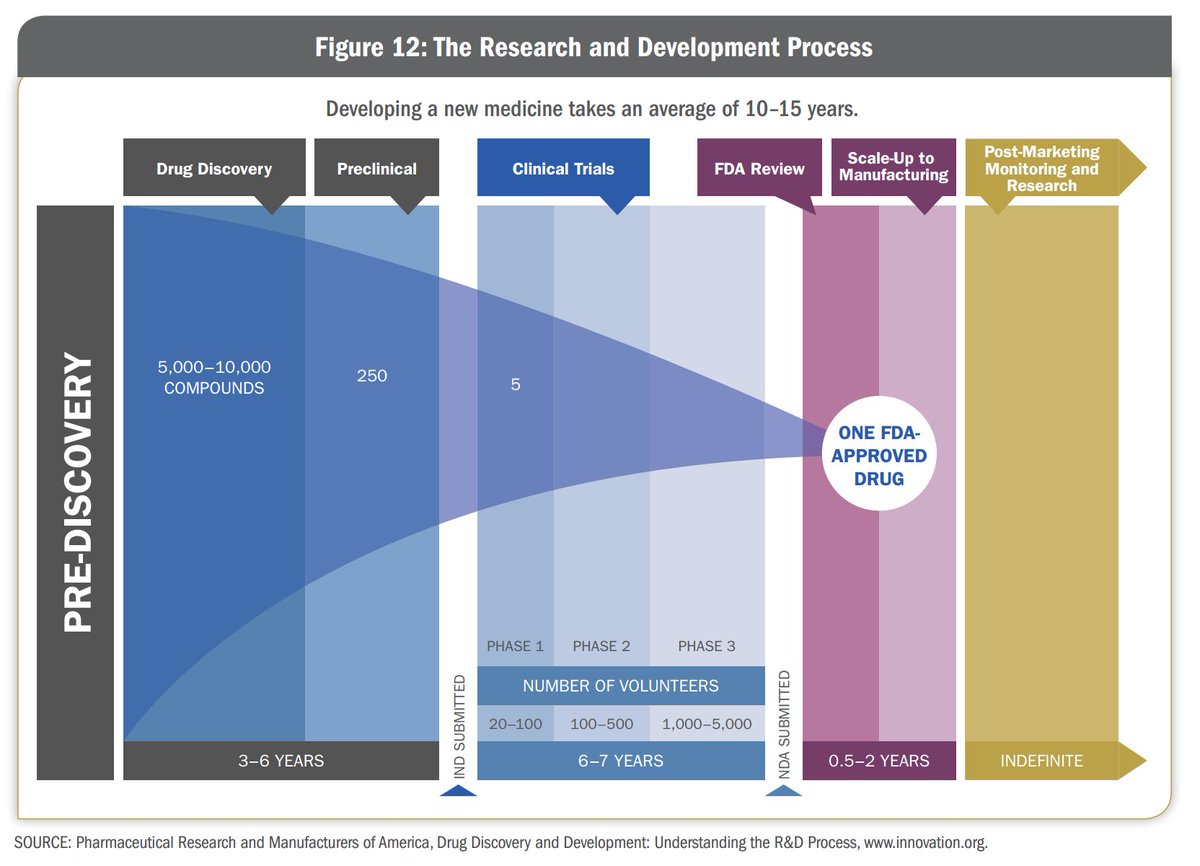
If the FDA approves the NDA, the company can manufacture and market the drugs to the public. But the testing does not end here. The drug now moves into Phase 4 testing.
Approximately 70% of the drugs move from Phase 1 to Phase 2, 33% move from Phase 2 to Phase 3, 25-30% move from Phase 3 to Phase 4. About 70-90% of drugs in Phase 4 are successful in staying in the market.
So where do the CROs and CDMOs come in? Contract research organizations help with the first 2 phases whereas Contract development and manufacturing organizations help with the last 2 phases
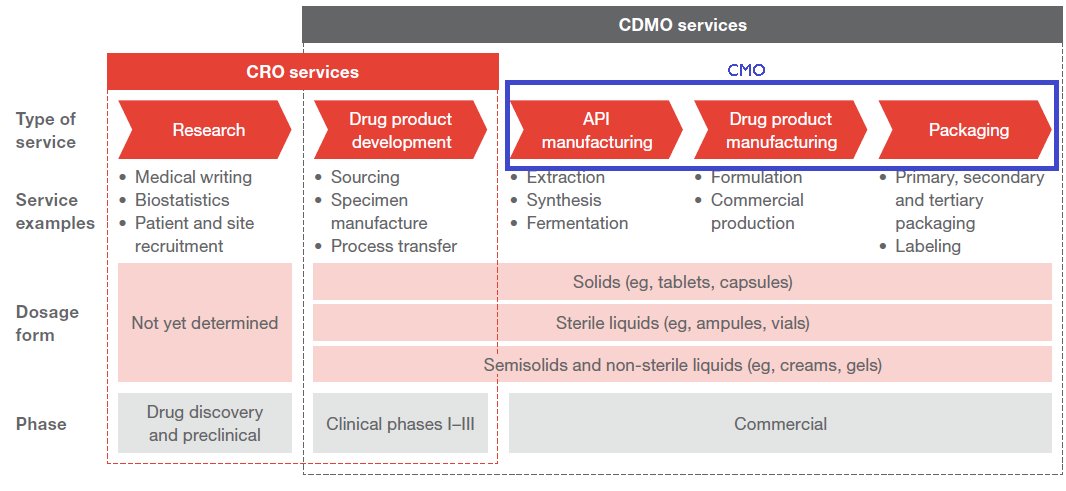
CROs support the innovators by providing services from drug discovery right up to commercialization. They provide services like target discovery, pre clinical trials, management of clinical trials, help with regulatory filings and pharmacovigilance(Phase 4).

Contract manufacturing organizations (CMO) on the other hand provide outsourced manufacturing solutions to the innovator. They are involved with the manufacturing of APIs and Formulations and oftentimes even involved with the final packaging of the product.
More from Value Educator
More from Uvlearnings
How many hospitals in India have globally comparable healthcare quality and high end molecular diagnostics, genomics diagnostics, AI/ML, Robotics for timely, accurate diagnosis and best possible prognosis, treatment at affordable cost?
— Sajal Kapoor (@unseenvalue) June 21, 2021
Any thoughts @tusharbohra @AdityaKhemka5 https://t.co/6Mhpu5Dru1
You May Also Like

I wish I had this... — don't excuse yourself. Forget about what you can't and focus on what you can.
Stop comparing yourself to others, come from the point of what you have, know and have: "I can... so I will do..!" #MyMindset

BTW this was an update of one of the previous tweets. And I'm continuing this thread today!
Focus only on positive things! These include what *you* have, know and can do. If you don't have, know or cannot do something either get it or ignore it. Don't think about it and don't use it as an excuse.
— Gleb Sabirzyanov (@zyumbik) October 17, 2018
I've been struggling to follow this principle for a long time. #MyMindset pic.twitter.com/SK5vtwHs3G
Do something for the long-term. Everything else is a distraction. 🛑 Nowadays I always check if the thing I'm doing aligns with my long-term plans. If not — that is probably not the best thing to do at the moment. #MyMindset
The only way to get more done is to have less to do. Eliminate your obligations, say "no" to things that are not important, stay minimal in what you do, focus. Being busy is not equal to getting things done. #MyMindset
What a weekend celebrating makers looks like.
A thread
👇Read on
Let's start with a crazy view of what @ProductHunt looked like on Sunday
Download image and upload
A top 7 with:
https://t.co/6gBjO6jXtB @Booligoosh
https://t.co/fwfKbQha57 @stephsmithio
https://t.co/LsSRNV9Jrf @anthilemoon
https://t.co/Fts7T8Un5M @J_Tabansi
Spotify Ctrl @shahroozme
https://t.co/37EoJAXEeG @kossnocorp
https://t.co/fMawYGlnro
If you want some top picks, see @deadcoder0904's thread,
We were going to have a go at doing this, but he nailed it.
It also comes with voting links 🖐so go do your
#24hrsstartup was an amazing event
— Akshay Kadam(A2K) \U0001f47b (@deadcoder0904) November 19, 2018
I never went to a hackathon but this just felt like one even though I was just watching \U0001f440
Everyone did great but there were a few startups that I personally loved \U0001f496
Some of my favorites are in the thread below\U0001f447
Over the following days the 24hr startup crew had more than their fair share of launches
Lots of variety: web, bots, extensions and even native apps
eg. @jordibruin with
\U0001f3a8\U0001f3c3\u200d\u2640\ufe0f DrawRun just launched on Product Hunt! Idea to App Store to Product Hunt in 68 hours!\u2070\u2070https://t.co/mxnLZ8FRSu
— Jordi Bruin (@jordibruin) November 20, 2018
Thanks for the motivation @thepatwalls @arminulrich @_feloidea
Here's the most useful #Factualist comparison pages #Thread 🧵

What is the difference between “pseudonym” and “stage name?”
Pseudonym means “a fictitious name (more literally, a false name), as those used by writers and movie stars,” while stage name is “the pseudonym of an entertainer.”
https://t.co/hT5XPkTepy #english #wiki #wikidiff
People also found this comparison helpful:
Alias #versus Stage Name: What’s the difference?
Alias means “another name; an assumed name,” while stage name means “the pseudonym of an entertainer.”
https://t.co/Kf7uVKekMd #Etymology #words
Another common #question:
What is the difference between “alias” and “pseudonym?”
As nouns alias means “another name; an assumed name,” while pseudonym means “a fictitious name (more literally, a false name), as those used by writers and movie
Here is a very basic #comparison: "Name versus Stage Name"
As #nouns, the difference is that name means “any nounal word or phrase which indicates a particular person, place, class, or thing,” but stage name means “the pseudonym of an