https://t.co/JSNC0mN7h2
Alright, folks. You may have seen that the FDA was in a fight with distilleries last week.
Let's unpack what happened, why it happened, and why it's now resulting in some extremely unusual behavior by HHS.
Thread below. Follow if you're interested.
https://t.co/JSNC0mN7h2
It basically sounded like "no good deed goes unpunished by the government." Reddit's take:
https://t.co/N75y14ZSvb
So let's look at what did happen, and you can decide for yourself where the blame lies.
Hand sanitizer is regulated as an over-the-counter (OTC) product. Under that regulatory approach, entities which adhere to an FDA "monograph" may market an OTC drug without obtaining explicit FDA approval.
Here's the sanitizer monograph: https://t.co/OicqzGEhvF
So on March 20, 2020, FDA released a new policy intended to help alleviate those shortages.
https://t.co/MobkmU2g5F
This policy was commonly understood to primarily target distillers of alcohol.
This requirement is longstanding and typically applies to traditional drug manufacturers.
- it allows the FDA to know the identities of all companies that make drugs
- Helps FDA conduct inspections and safety surveillance
- Indicates which companies owe fees to FDA
These fees are used to help fund the FDA, and make up more than half of its overall budget.
- Application fees, assessed each time a company wants FDA to review a new drug or medical product, and
- Facility fees, assessed on an annual basis and used to offset the costs of inspections and routine regulatory work.
But never for nonprescription (OTC) drugs.
As a result, OTC companies had to register, but didn't have to pay fees.
March 20, 2020. That's when FDA released its guidance document.
A week later, on March 27th, Congress passed the Coronavirus Aid, Relief, and Economic Security (CARES) Act.
This is where things get interesting.
It also established a user fee program called the OTC Monograph Drug User Fee Program (OMUFA). https://t.co/A6ued6pmRo
Here's the relevant explanation from my writeup for AgencyIQ:
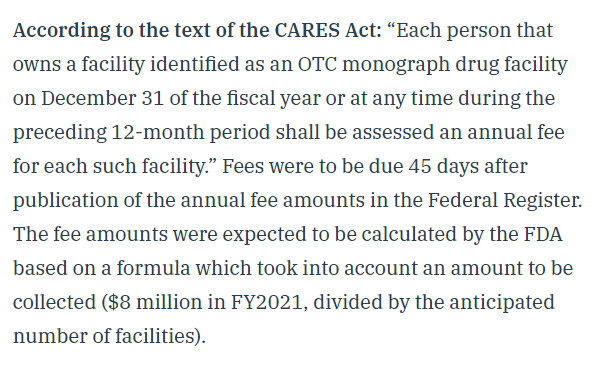
That changed on December 29, when FDA announced the OMUFA fees for FY2021: https://t.co/zkcfavN8xs
And because distilleries had been required to register, that would now include distilleries, too.
Some had even donated these supplies, meaning they would have lost even more $.
https://t.co/ekIkzjs3lf

This actually has a pretty simple answer: Congress.
Basically: Congress gives FDA a formula, and tells it to use the formula to determine the fees. (This formula is usually signed off by industry groups prior to passage, as it was here).
Those *do* exist for other user fee programs.
Given that it had 45 days to do so, that seemed like a possible timeline.
https://t.co/jM5Ox8BjVr


https://t.co/F1mqF1zZ07
Proof: https://t.co/ZflKfKtS6J
The order: https://t.co/TWIsTB3F8n
OTC groups I spoke with are... not pleased.
It applies a basic formula to determine what the annual fee should be by taking a base amount mandated by Congress and then dividing by the number of active facilities.
Alternatively, anything that HHS doesn’t like is “significant” and therefore a “legislative rule.”
The only online version I could find was here: https://t.co/DpaKdsQp24
You can read it here: https://t.co/TWIsTB3F8n
Folks, this is not how government normally works. Now, this isn’t the first time that FDA and HHS have been at odds in recent weeks…
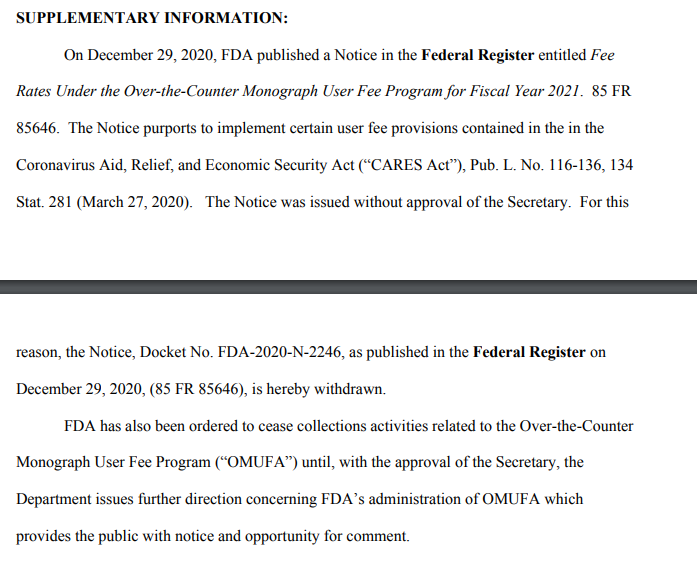
See my colleagues @DanDiamond and @DavidALim’s story: https://t.co/bf3E3oRAOc
So withdrawing them just punts the issue down the road.
- Public Health Emergency waiver
- First-time-filer waiver
- Small business waiver
Easy fixes that wouldn’t be difficult to implement and are common to other programs.
More from News
.@SenatorTester said that in order to take on Trump, you have to \u201cpunch him in the face\u201d pic.twitter.com/7NPJ3MOk2i
— Tom Elliott (@tomselliott) January 8, 2021
@mjs_DC 2)
Earlier this year, @SpeakerPelosi said of getting active in politics, \u201cWhen you\u2019re in the arena, you have to be ready to take a punch and you have to be ready to throw a punch\u201d pic.twitter.com/xe4U5ElFCW
— Tom Elliott (@tomselliott) January 8, 2021
@mjs_DC
In an interview w/ Joy Ann Reid, Rep. Waters then doubled down on her call to be \u201cmore confrontational\u201d w/ Republicans pic.twitter.com/SnXfHc6hJg
— Tom Elliott (@tomselliott) January 8, 2021
@mjs_DC
.@RepMaxineWaters told activists that \u201cGod is in our side\u201d and urged her followers be increasingly confrontational members of the Trump Administration pic.twitter.com/W9X4A7jAXN
— Tom Elliott (@tomselliott) January 8, 2021
@mjs_DC 5)
.@EricHolder told liberal activists that Michelle Obama was wrong; \u201cWhen they go low, we go high. No. No. When they go low, we kick them." pic.twitter.com/tWQfyLr2Cu
— Tom Elliott (@tomselliott) January 8, 2021
You May Also Like
Who are these chuds?
Patriot Front broke away from white nationalist org Vanguard America following #unitetheright in #charlottesville after James Alex Fields was seen with a VA shield before driving his car into a crowd, murdering Heather Heyer & injuring dozens of others
Syed Robbie Javid a.k.a. Sayed Robbie Javid or Robbie Javid of Alexandria,
Happy Monday everyone :-) Let's ring in September by reacquainting ourselves with Virginia neo-Nazi and NSC Dixie affiliate Sayed "Robbie" Javid, now known by "Reform the States". Robbie is an explicitly genocidal neo-Nazi, so lets get to know him a bit better!
— Garfield but Anti-Fascist (@AntifaGarfield) August 31, 2020
CW on this thread pic.twitter.com/3gzxrIo9HD
Antoine Bernard Renard (a.k.a. “Charlemagne MD” on Discord) from Rockville, MD.
https://t.co/ykEjdZFDi6

Brandon Troy Higgs, 25, from Reisterstown,






























