This is a limited point about availability of efficacy data for vaccines under development in the context of the approval for CovidShield and Covaxin in India.
There have been many so-called experts on the idiotbox opining about apparent availability of P III data which 1/n
Here is one set of efficacy data post the interim analysis of a mRNA vaccine.
Source: https://t.co/BAPnP3PxEb
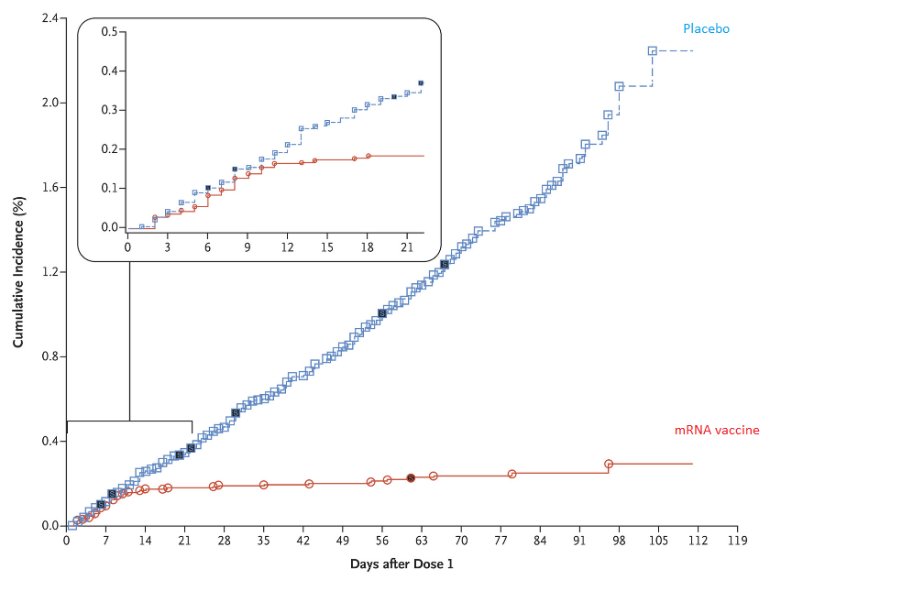
How does the SEC, or the sponsor of these studies, or the experts who are offering their opinion liberally on the idiotbox know what the efficacy is
A. Do they know if the blind was broken for the bridging study and the Phase III study?
B. If so, can they produce data like the one above showing how many subjects who were infected were
And if they cannot answer this question, then ask the following question:
C. In the absence of efficacy data, how does one claim that the vaccine candidate is effective?
D. Do they agree that therapeutic candidates ought to be approved
More from Health
You May Also Like
On the occasion of youtube 20k and Twitter 70k members
A small tribute/gift to members
Screeners
technical screeners - intraday and positional both
before proceeding - i have helped you , can i ask you so that it can help someone else too
thank you
positional one
run - find #stock - draw chart - find levels
1- Stocks closing daily 2% up from 5 days
https://t.co/gTZrYY3Nht
2- Weekly breakout
https://t.co/1f4ahEolYB
3- Breakouts in short term
https://t.co/BI4h0CdgO2
4- Bullish from last 5
intraday screeners
5- 15 minute Stock Breakouts
https://t.co/9eAo82iuNv
6- Intraday Buying seen in the past 15 minutes
https://t.co/XqAJKhLB5G
7- Stocks trading near day's high on 5 min chart with volume BO intraday
https://t.co/flHmm6QXmo
Thank you
A small tribute/gift to members
Screeners
technical screeners - intraday and positional both
before proceeding - i have helped you , can i ask you so that it can help someone else too
thank you
positional one
run - find #stock - draw chart - find levels
1- Stocks closing daily 2% up from 5 days
https://t.co/gTZrYY3Nht
2- Weekly breakout
https://t.co/1f4ahEolYB
3- Breakouts in short term
https://t.co/BI4h0CdgO2
4- Bullish from last 5
intraday screeners
5- 15 minute Stock Breakouts
https://t.co/9eAo82iuNv
6- Intraday Buying seen in the past 15 minutes
https://t.co/XqAJKhLB5G
7- Stocks trading near day's high on 5 min chart with volume BO intraday
https://t.co/flHmm6QXmo
Thank you