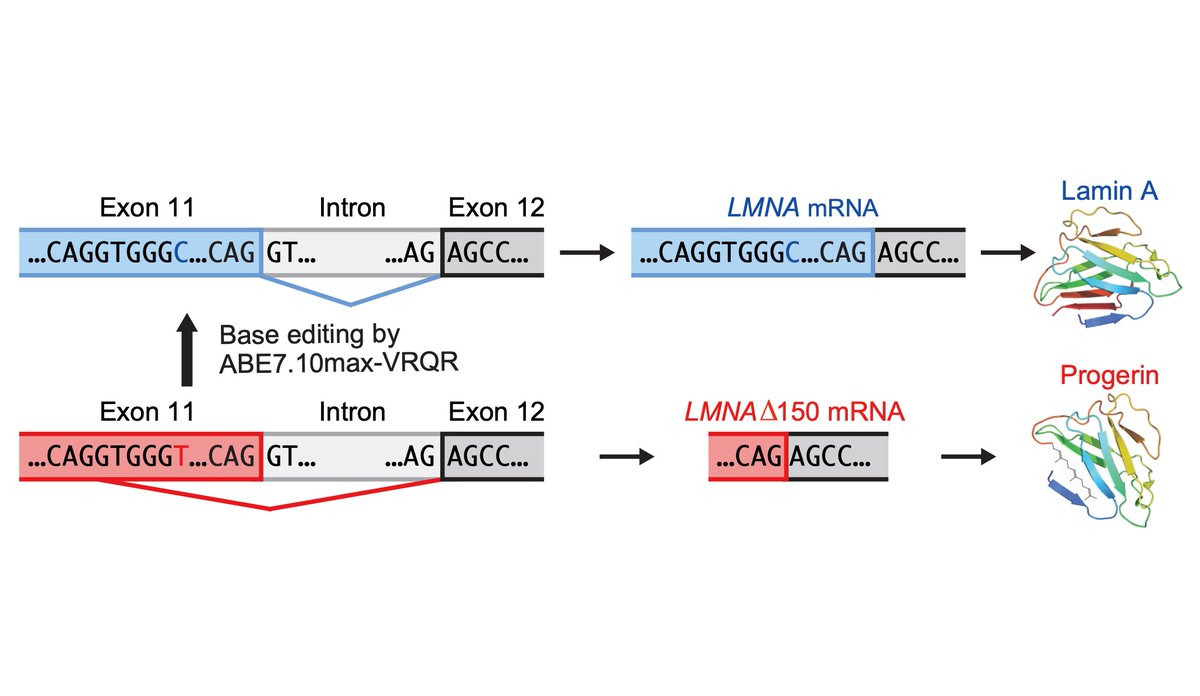
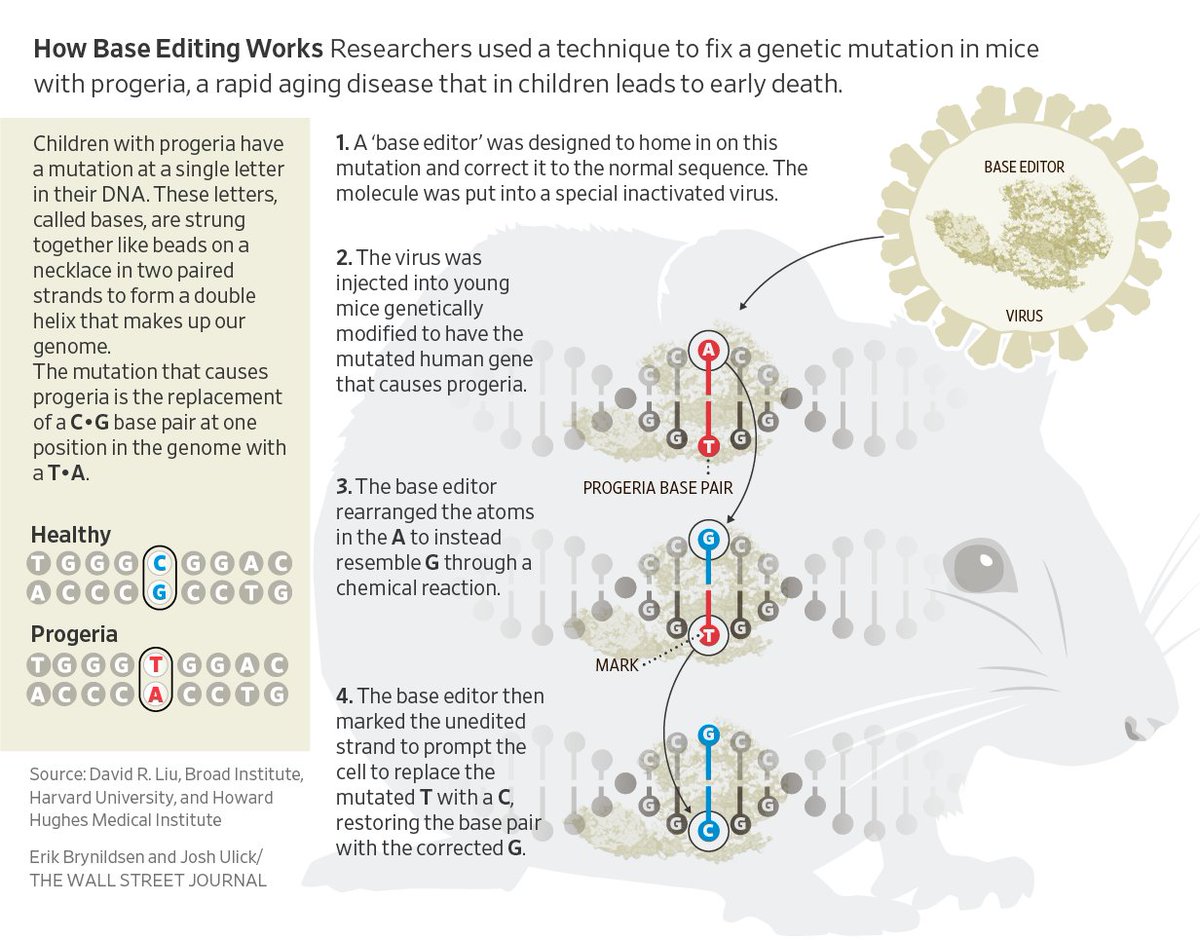
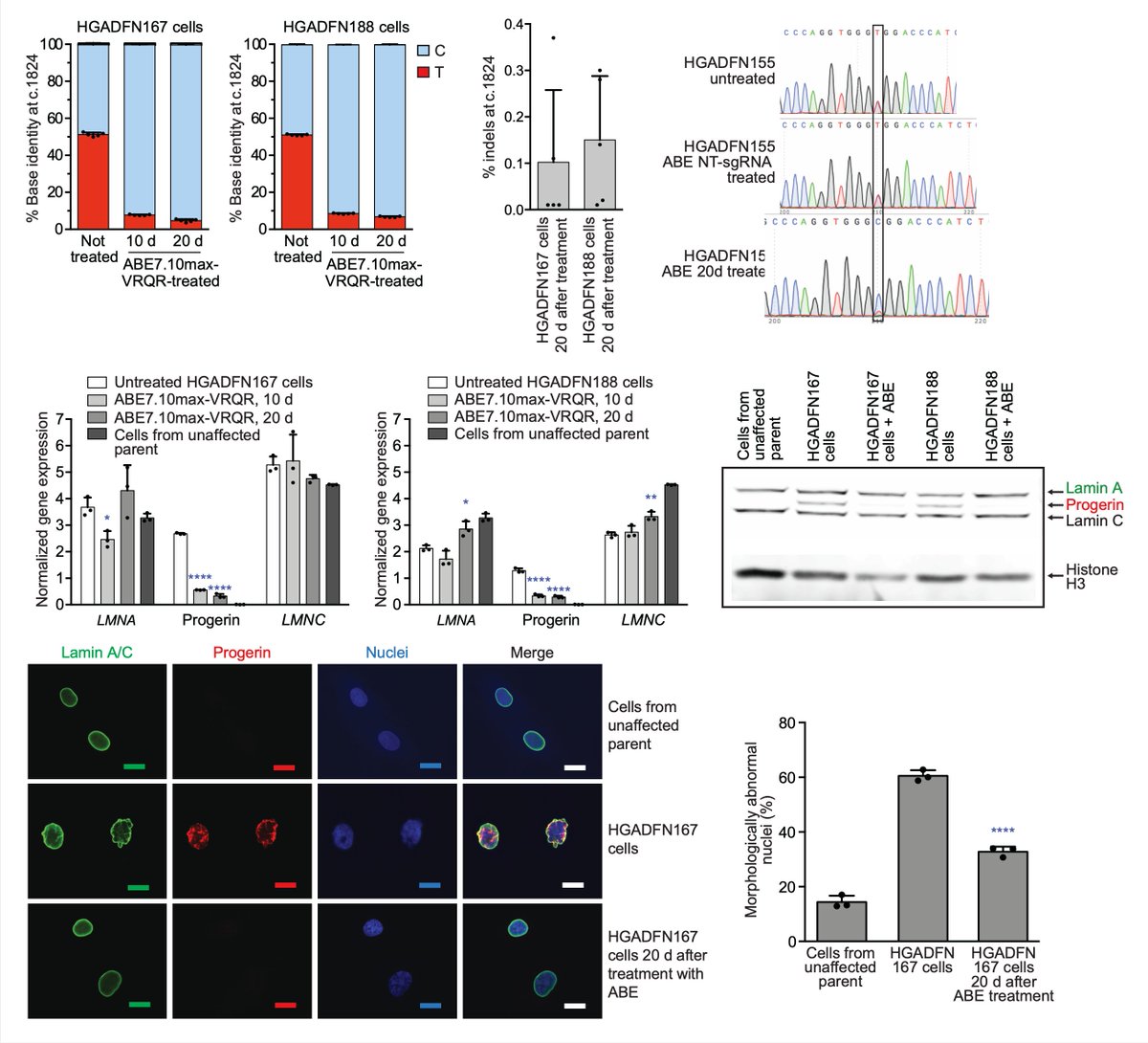
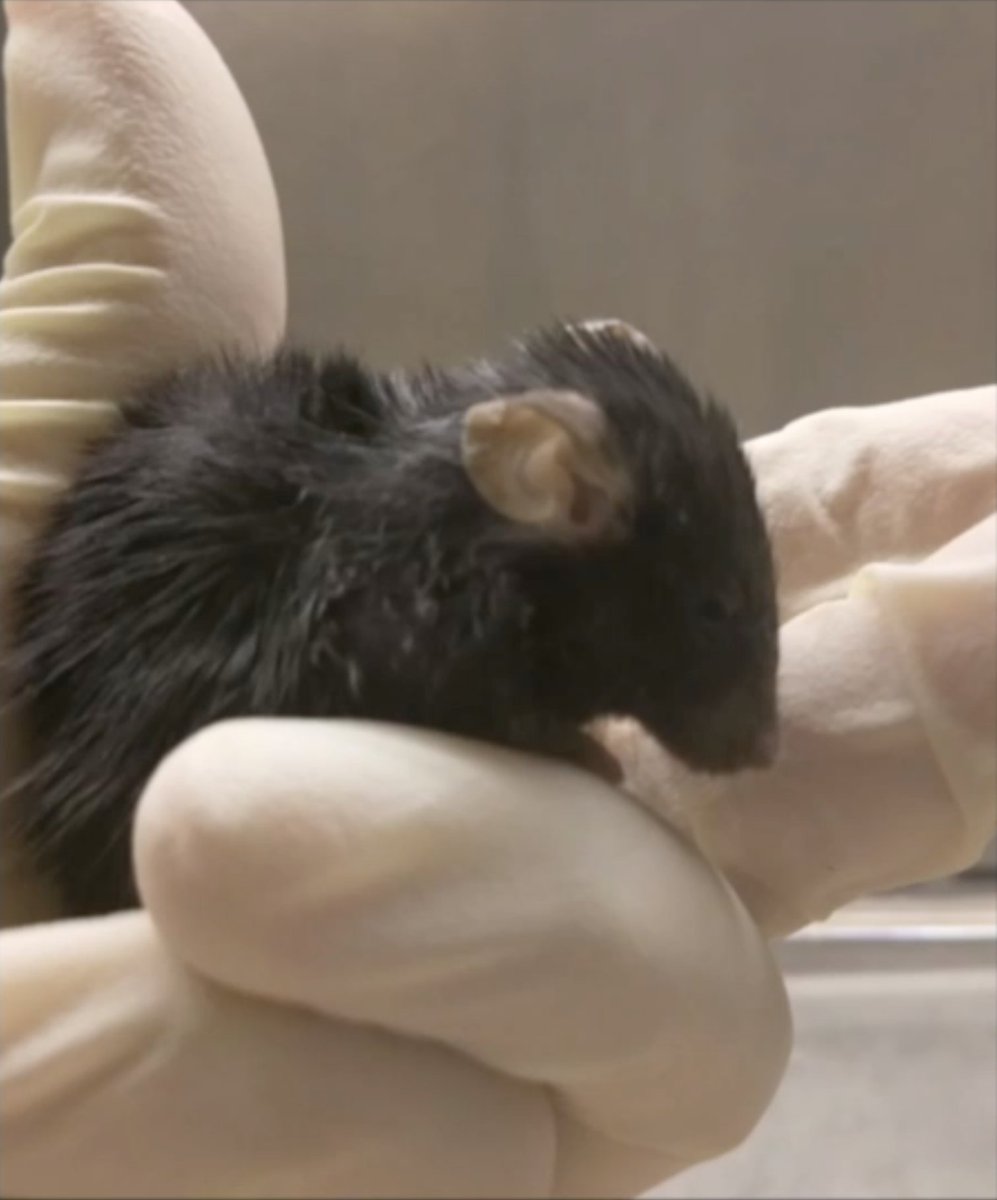
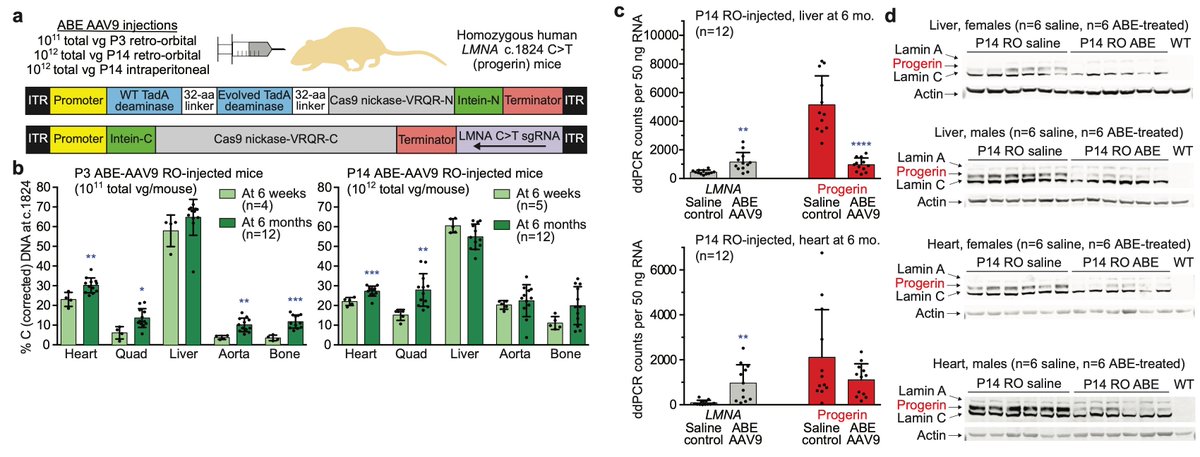
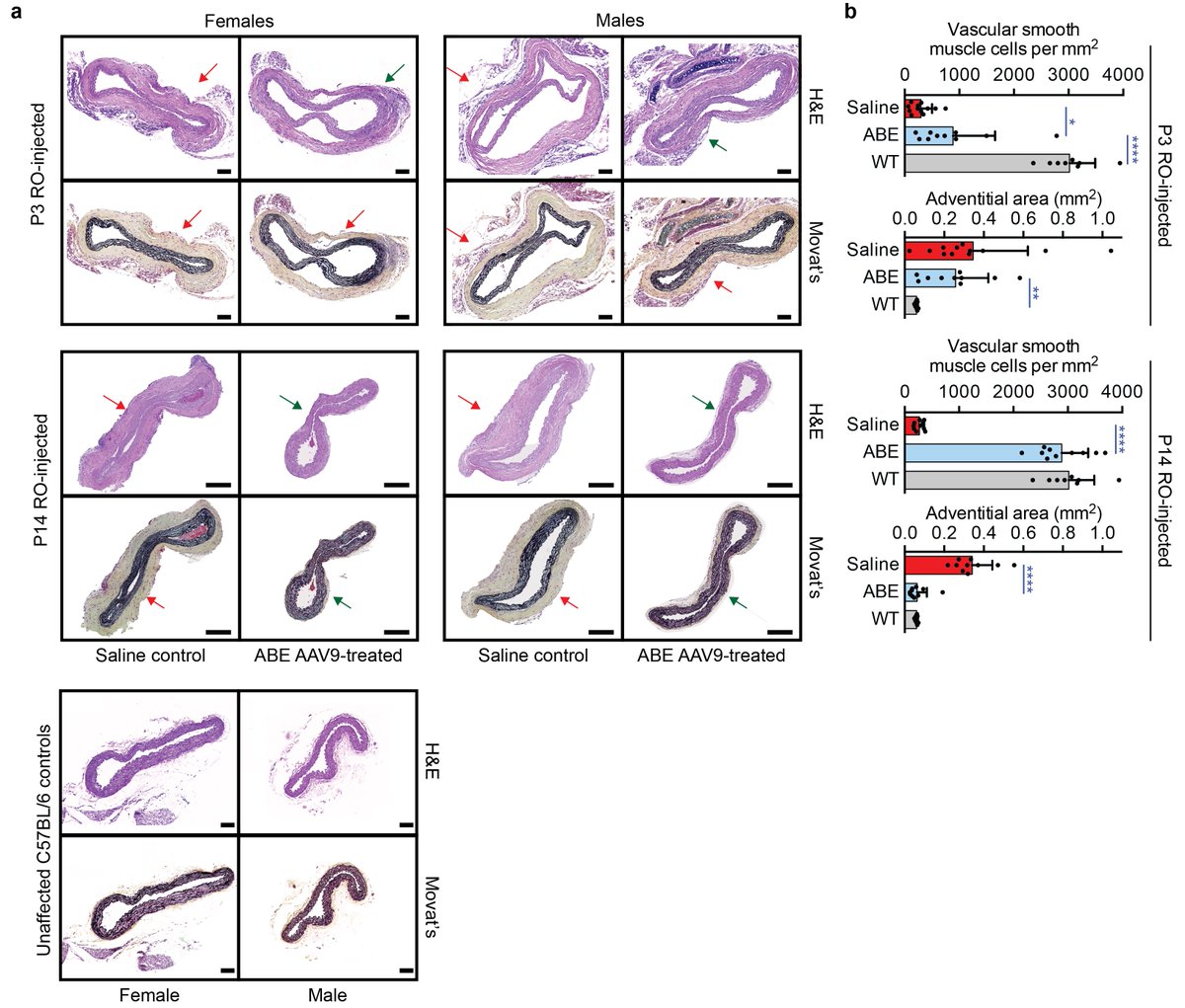
Today we report the use of base editing in patient-derived cells and in mice to correct the most common cause of progeria, the devastating rapid aging disease. Progeria is typically caused by a dominant negative C•G-to-T•A point mutation in LMNA. 1/11 https://t.co/O7dkEYpndg

More from Science
💥💥 Situation Update, Dec. 7th – DNI John Ratcliffe, the bogus science of PCR testing and China’s GMO super soldiers
✅ I cover the bogus science behind PCR testing, explaining from a lab science point of view why no PCR instrument can “quantify” anything,
[M. Adams]

1. whether it’s a coronavirus viral load or the percentage of a food that’s GMO. In fact, literally all the tests currently conducted with PCR equipment are scientifically invalid when it comes to diagnosing illness or determining infectiousness. The sample acquisition used for
2. PCR tests — nasal swabs — aren’t even standardized! (100% bogus junk science).
After covering PCR tests, today’s update then goes into detail about Director of National Intelligence (DNI) John Ratcliffe, pointing out that he will be issuing a report on foreign interference
3. in U.S. elections on or before Dec. 18th. If this report confirms the existence of foreign interference that was capable of altering the outcome of the election, it gives President Trump full justification to declare the election null and void and dispatch military troops
4. to seize all ballots and hold a new count under military authority.
👉 Podcast notes and sources:
The office of military commissions has cleared its calendar for December:
https://t.co/u4nFRiUj8m
US military STOCKPILED Pfizer’s mRNA vaccineBEFORE it was approved by theFDA
✅ I cover the bogus science behind PCR testing, explaining from a lab science point of view why no PCR instrument can “quantify” anything,
[M. Adams]

1. whether it’s a coronavirus viral load or the percentage of a food that’s GMO. In fact, literally all the tests currently conducted with PCR equipment are scientifically invalid when it comes to diagnosing illness or determining infectiousness. The sample acquisition used for
2. PCR tests — nasal swabs — aren’t even standardized! (100% bogus junk science).
After covering PCR tests, today’s update then goes into detail about Director of National Intelligence (DNI) John Ratcliffe, pointing out that he will be issuing a report on foreign interference
3. in U.S. elections on or before Dec. 18th. If this report confirms the existence of foreign interference that was capable of altering the outcome of the election, it gives President Trump full justification to declare the election null and void and dispatch military troops
4. to seize all ballots and hold a new count under military authority.
👉 Podcast notes and sources:
The office of military commissions has cleared its calendar for December:
https://t.co/u4nFRiUj8m
US military STOCKPILED Pfizer’s mRNA vaccineBEFORE it was approved by theFDA
So it turns out that an organization I thought was doing good work, the False Memory Syndrome Foundation (associated with Center for Inquiry, James Randi, and Martin Gardner) was actually caping for pedophiles. Uhhhh oops?
Since this, bizarrely, turned out to be one of my longest videos ever (??) here's a quick thread to sum it up for those of you like myself with short attention spans. 1/10
In the '90s the False Memory Syndrome Foundation was founded to call attention to the problem of adults suddenly "remembering" child abuse that never actually happened, often under hypnosis. Skeptics like James Randi & Martin Gardner joined their board. 2/10
A new article reveals that the FMSF was founded by parents who had been credibly and PRIVATELY accused of molestation by their now-adult daughter. They publicized the accusation, destroyed the daughter's reputation, and started the foundation. 3/10
The FMSF assumed any accused pedo who joined was innocent, saying "We are a good-looking bunch of people, graying hair, well dressed, healthy, smiling; just about every person who has attended is someone you would surely find interesting and want to count as a friend" 😬 4/10

I was Wrong about False Memories: Satanic Panic, Pedophiles, Ted Bundy, and the Lost in the Mall Studies https://t.co/6XKTfGOqwl
— skepchicks (@skepchicks) January 15, 2021
Since this, bizarrely, turned out to be one of my longest videos ever (??) here's a quick thread to sum it up for those of you like myself with short attention spans. 1/10
In the '90s the False Memory Syndrome Foundation was founded to call attention to the problem of adults suddenly "remembering" child abuse that never actually happened, often under hypnosis. Skeptics like James Randi & Martin Gardner joined their board. 2/10
A new article reveals that the FMSF was founded by parents who had been credibly and PRIVATELY accused of molestation by their now-adult daughter. They publicized the accusation, destroyed the daughter's reputation, and started the foundation. 3/10
The FMSF assumed any accused pedo who joined was innocent, saying "We are a good-looking bunch of people, graying hair, well dressed, healthy, smiling; just about every person who has attended is someone you would surely find interesting and want to count as a friend" 😬 4/10

You May Also Like
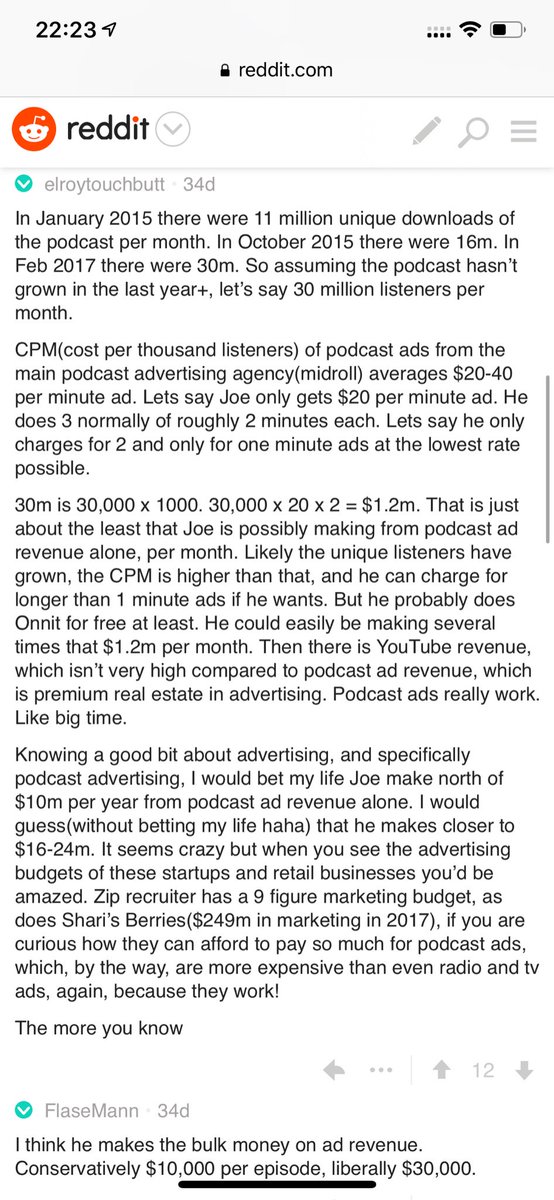
Joe Rogan's podcast is now is listened to 1.5+ billion times per year at around $50-100M/year revenue.
Independent and 100% owned by Joe, no networks, no middle men and a 100M+ people audience.
👏
https://t.co/RywAiBxA3s
Joe is the #1 / #2 podcast (depends per week) of all podcasts
120 million plays per month source https://t.co/k7L1LfDdcM
https://t.co/aGcYnVDpMu
Independent and 100% owned by Joe, no networks, no middle men and a 100M+ people audience.
👏
https://t.co/RywAiBxA3s
Joe is the #1 / #2 podcast (depends per week) of all podcasts
120 million plays per month source https://t.co/k7L1LfDdcM

https://t.co/aGcYnVDpMu

So friends here is the thread on the recommended pathway for new entrants in the stock market.
Here I will share what I believe are essentials for anybody who is interested in stock markets and the resources to learn them, its from my experience and by no means exhaustive..
First the very basic : The Dow theory, Everybody must have basic understanding of it and must learn to observe High Highs, Higher Lows, Lower Highs and Lowers lows on charts and their
Even those who are more inclined towards fundamental side can also benefit from Dow theory, as it can hint start & end of Bull/Bear runs thereby indication entry and exits.
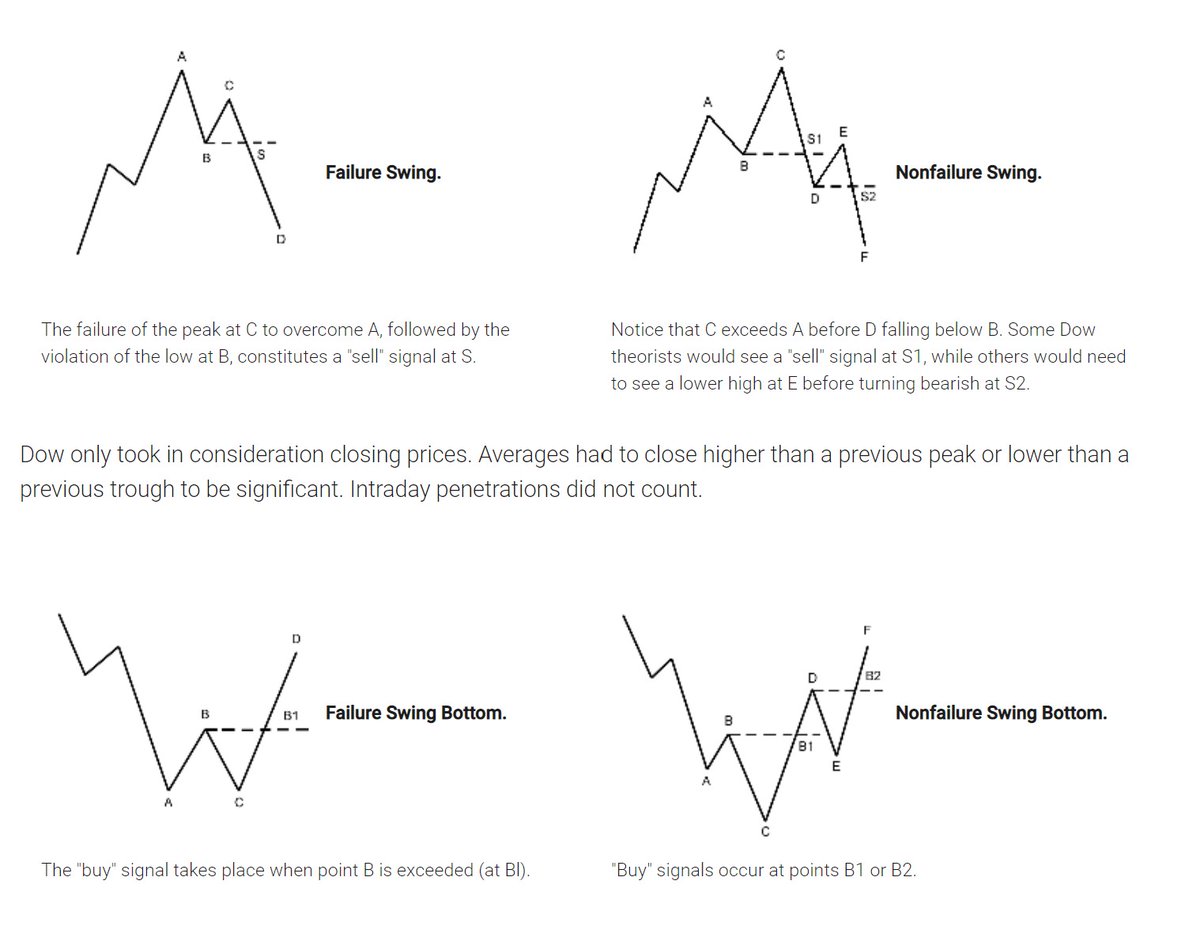
Next basic is Wyckoff's Theory. It tells how accumulation and distribution happens with regularity and how the market actually
Dow theory is old but
Here I will share what I believe are essentials for anybody who is interested in stock markets and the resources to learn them, its from my experience and by no means exhaustive..
First the very basic : The Dow theory, Everybody must have basic understanding of it and must learn to observe High Highs, Higher Lows, Lower Highs and Lowers lows on charts and their
Even those who are more inclined towards fundamental side can also benefit from Dow theory, as it can hint start & end of Bull/Bear runs thereby indication entry and exits.

Next basic is Wyckoff's Theory. It tells how accumulation and distribution happens with regularity and how the market actually
Dow theory is old but
Old is Gold....
— Professor (@DillikiBiili) January 23, 2020
this Bharti Airtel chart is a true copy of the Wyckoff Pattern propounded in 1931....... pic.twitter.com/tQ1PNebq7d