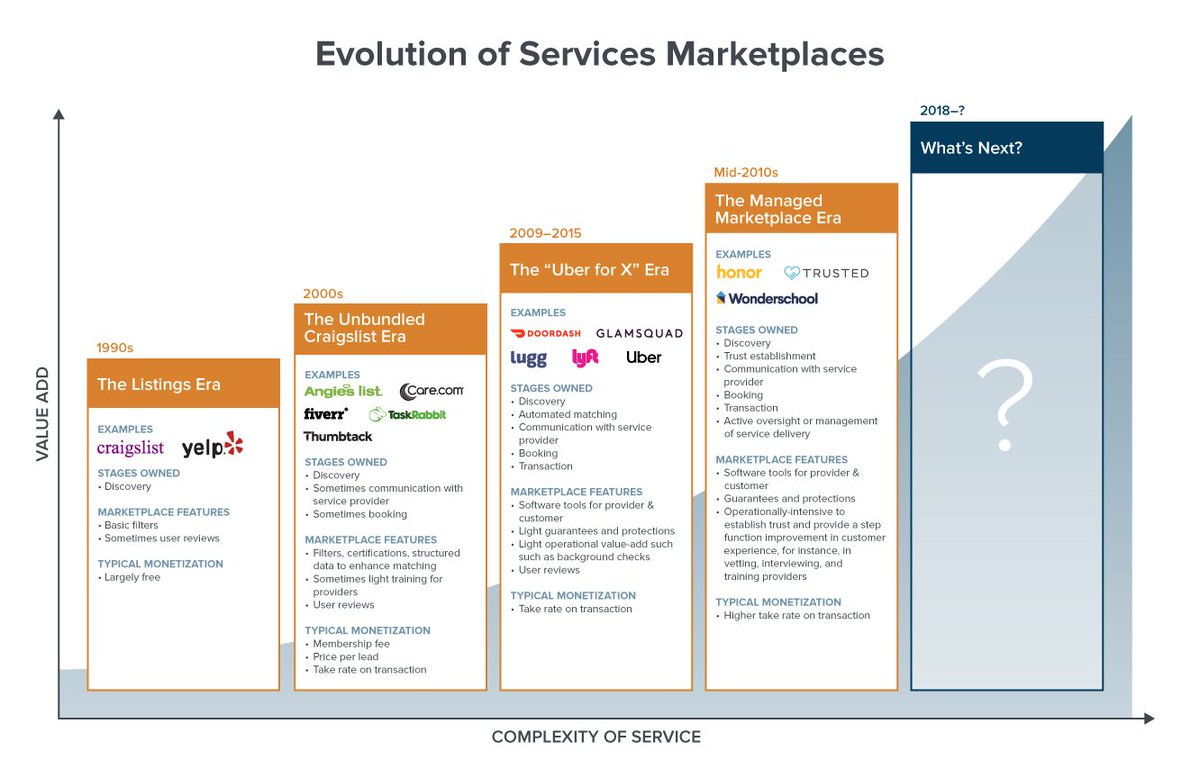
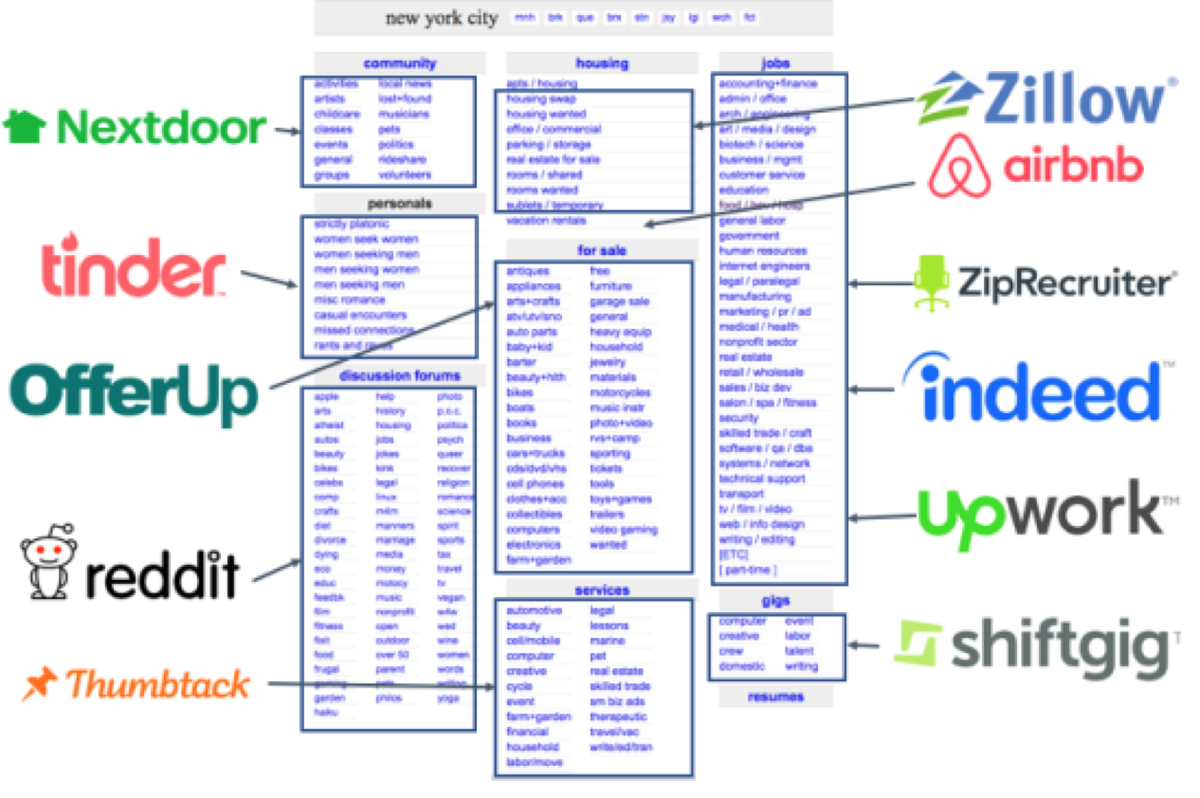
1/Part 2 on #diabetes #prevention based on the @TheLancet diabetes commission report.
Individual level prevention of type 2 diabetes (T2D) gets a lot of attention. Part 2 reflect this - but don’t worry, there will be more on the other levels in part 3..
Let’s get going..
https://t.co/yGEYwJZrSj
More from Health
This is a limited point about availability of efficacy data for vaccines under development in the context of the approval for CovidShield and Covaxin in India.
There have been many so-called experts on the idiotbox opining about apparent availability of P III data which 1/n
2/n apparently the SEC had access to based on which it "supposedly" approved Covaxin. Another argument that is prevalent is other regulators (US FDA and MHRA) also approved vaccines based on P II data alone. Let me give you a few facts so that you can make your own decision.
3/n The protocols for both mRNA vaccines are publicly available. You can check. Both protocols *define* when the interim analysis will be done. This is not subjective. They clearly define how many infections need to be documented before the Data Safety Monitoring Board meets.
4/n Find the protocols for the bridging study for CovidShield and Covaxin and look for a similar milestone.
Here is one set of efficacy data post the interim analysis of a mRNA vaccine.
Source: https://t.co/BAPnP3PxEb
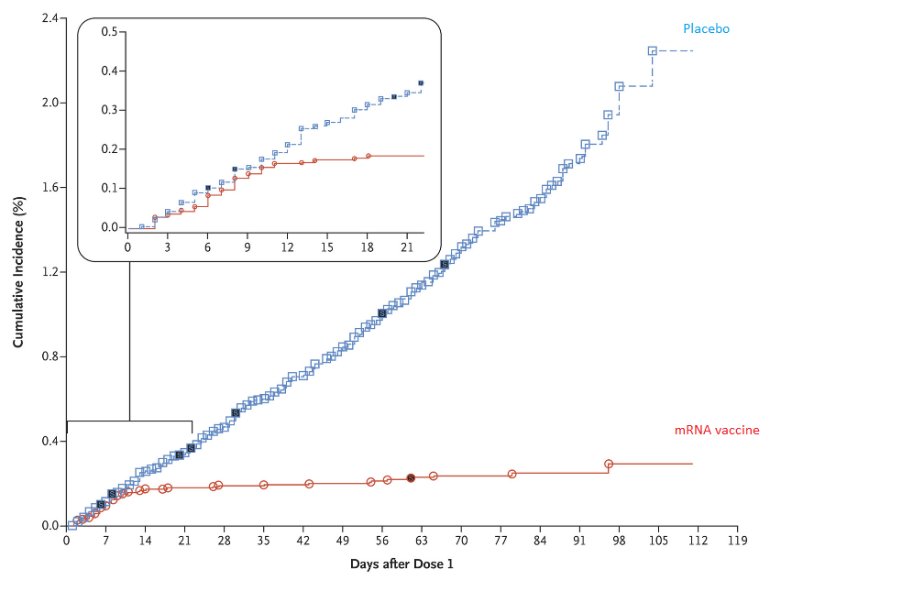
5/n This data was analyzed post the interim analysis where the blind was broken by the DSMB. Now ask yourself this question:
How does the SEC, or the sponsor of these studies, or the experts who are offering their opinion liberally on the idiotbox know what the efficacy is
There have been many so-called experts on the idiotbox opining about apparent availability of P III data which 1/n
2/n apparently the SEC had access to based on which it "supposedly" approved Covaxin. Another argument that is prevalent is other regulators (US FDA and MHRA) also approved vaccines based on P II data alone. Let me give you a few facts so that you can make your own decision.
3/n The protocols for both mRNA vaccines are publicly available. You can check. Both protocols *define* when the interim analysis will be done. This is not subjective. They clearly define how many infections need to be documented before the Data Safety Monitoring Board meets.
4/n Find the protocols for the bridging study for CovidShield and Covaxin and look for a similar milestone.
Here is one set of efficacy data post the interim analysis of a mRNA vaccine.
Source: https://t.co/BAPnP3PxEb

5/n This data was analyzed post the interim analysis where the blind was broken by the DSMB. Now ask yourself this question:
How does the SEC, or the sponsor of these studies, or the experts who are offering their opinion liberally on the idiotbox know what the efficacy is
You May Also Like
My top 10 tweets of the year
A thread 👇
https://t.co/xj4js6shhy
https://t.co/b81zoW6u1d
https://t.co/1147it02zs
https://t.co/A7XCU5fC2m
A thread 👇
https://t.co/xj4js6shhy
Entrepreneur\u2019s mind.
— James Clear (@JamesClear) August 22, 2020
Athlete\u2019s body.
Artist\u2019s soul.
https://t.co/b81zoW6u1d
When you choose who to follow on Twitter, you are choosing your future thoughts.
— James Clear (@JamesClear) October 3, 2020
https://t.co/1147it02zs
Working on a problem reduces the fear of it.
— James Clear (@JamesClear) August 30, 2020
It\u2019s hard to fear a problem when you are making progress on it\u2014even if progress is imperfect and slow.
Action relieves anxiety.
https://t.co/A7XCU5fC2m
We often avoid taking action because we think "I need to learn more," but the best way to learn is often by taking action.
— James Clear (@JamesClear) September 23, 2020