[Thread]: Old school "medicine" ads obviously created by aliens.
Let's begin with cocaine toothache drops, for only 15 cents.

"It is simply iron and Quinine in a tasteless form".

Don't forget to use it when you need to clean and restore color to the carpet.



The cat in a dress skipping rope is important.

More from Marina Amaral
More from Health
1/16
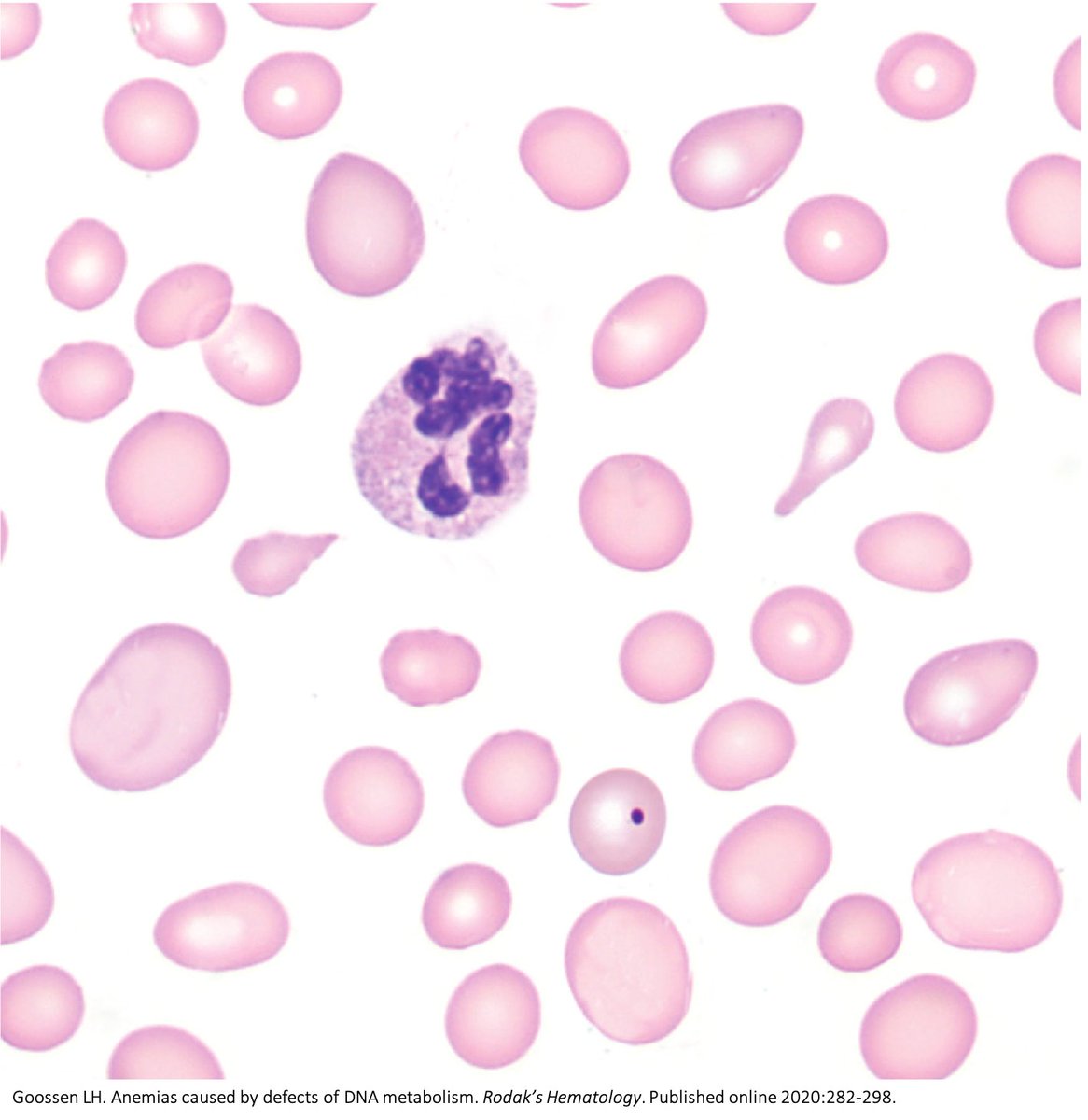
Why do B12 and folate deficiencies lead to HUGE red blood cells?
And, if the issue is DNA synthesis, why are red blood cells (which don't have DNA) the key cell line affected?
For answers, we'll have to go back a few billion years.
2/
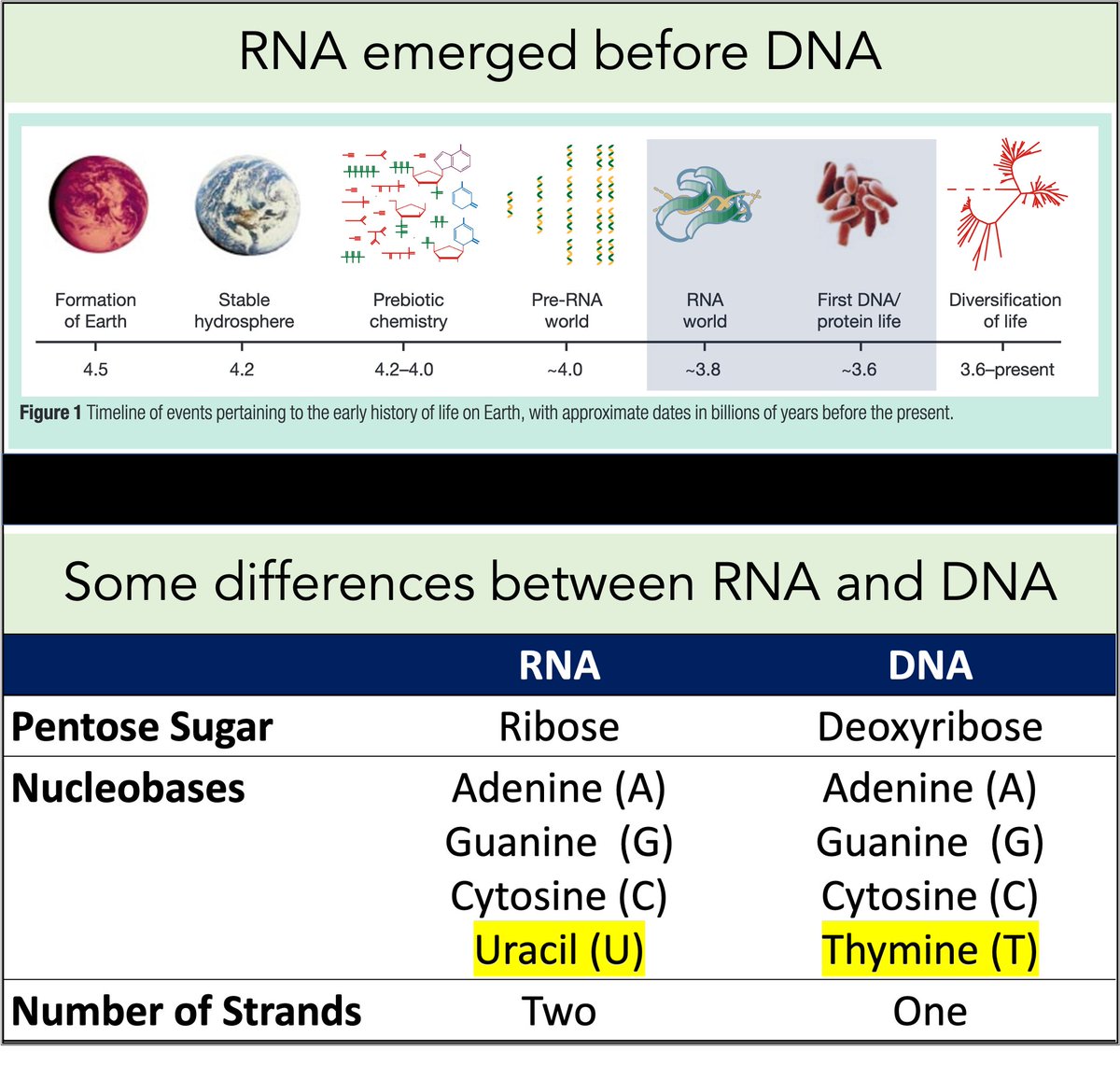
RNA came first. Then, ~3-4 billion years ago, DNA emerged.
Among their differences:
🔹RNA contains uracil
🔹DNA contains thymine
But why does DNA contains thymine (T) instead of uracil (U)?
https://t.co/XlxT6cLLXg
3/
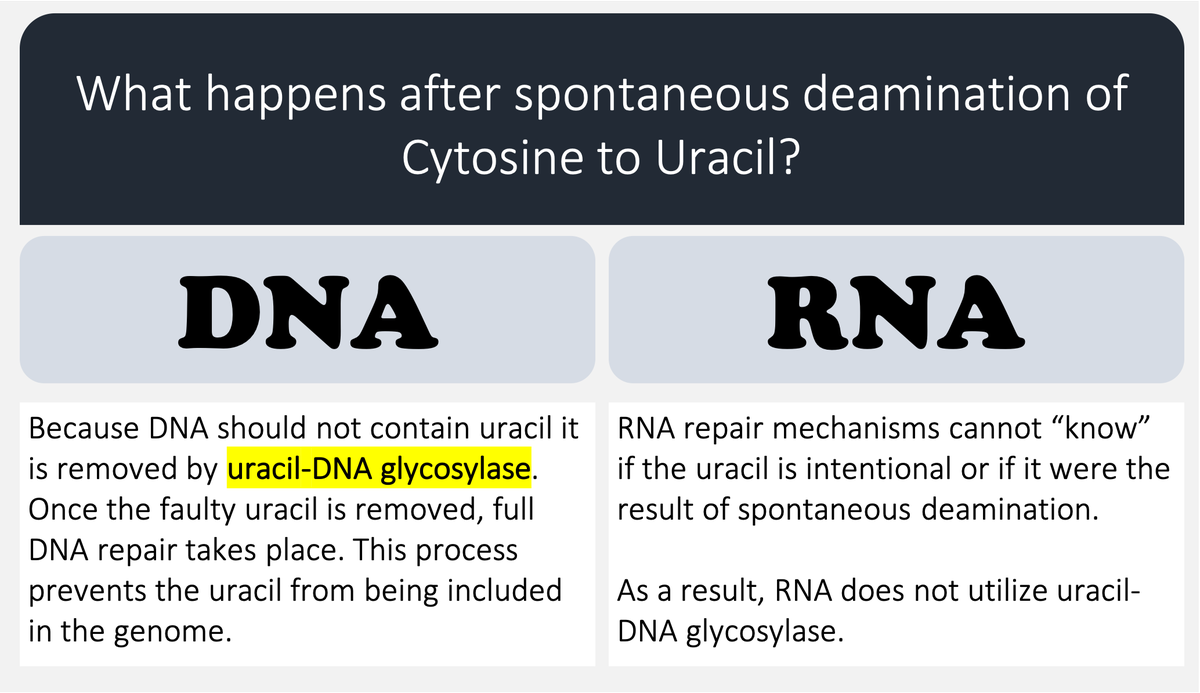
🔑Cytosine (C) can undergo spontaneous deamination to uracil (U).
In the RNA world, this meant that U could appear intensionally or unintentionally. This is clearly problematic. How can you repair RNA when you can't tell if something is an error?
https://t.co/bIZGviHBUc
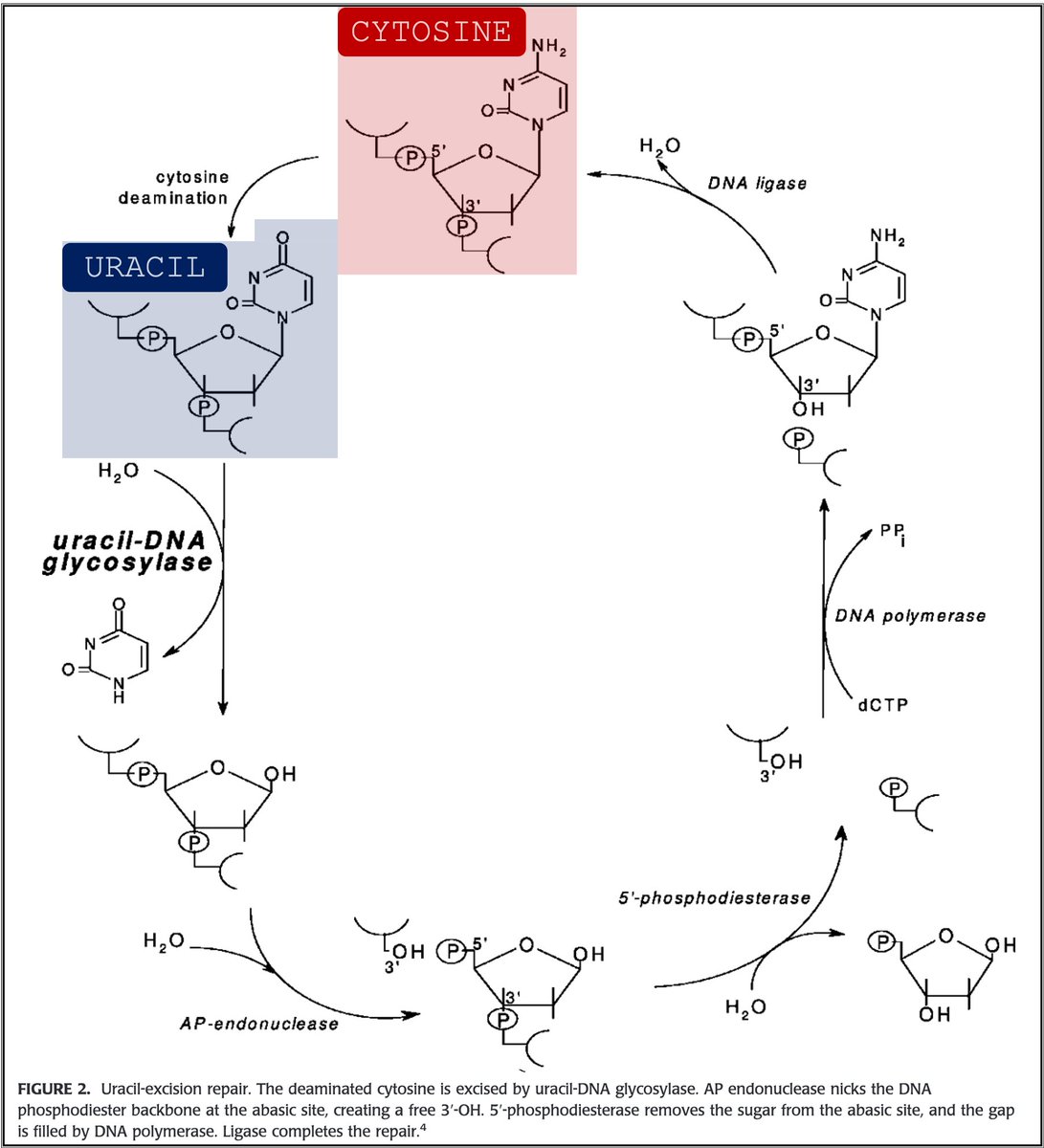
4/
DNA's use of T instead of U means that spontaneous C → U deamination can be corrected without worry that an intentional U is being removed.
DNA requires greater stability than RNA so the transition to a thymine-based structure was beneficial.
https://t.co/bIZGviHBUc
5/
Let's return to megaloblastic anemia secondary to B12 or folate deficiency.
When either is severely deficient deoxythymidine monophosphate (dTMP*) production is hindered. With less dTMP, DNA synthesis is abnormal.
[*Note: thymine is the base in dTMP]
https://t.co/AnDUtKkbZh
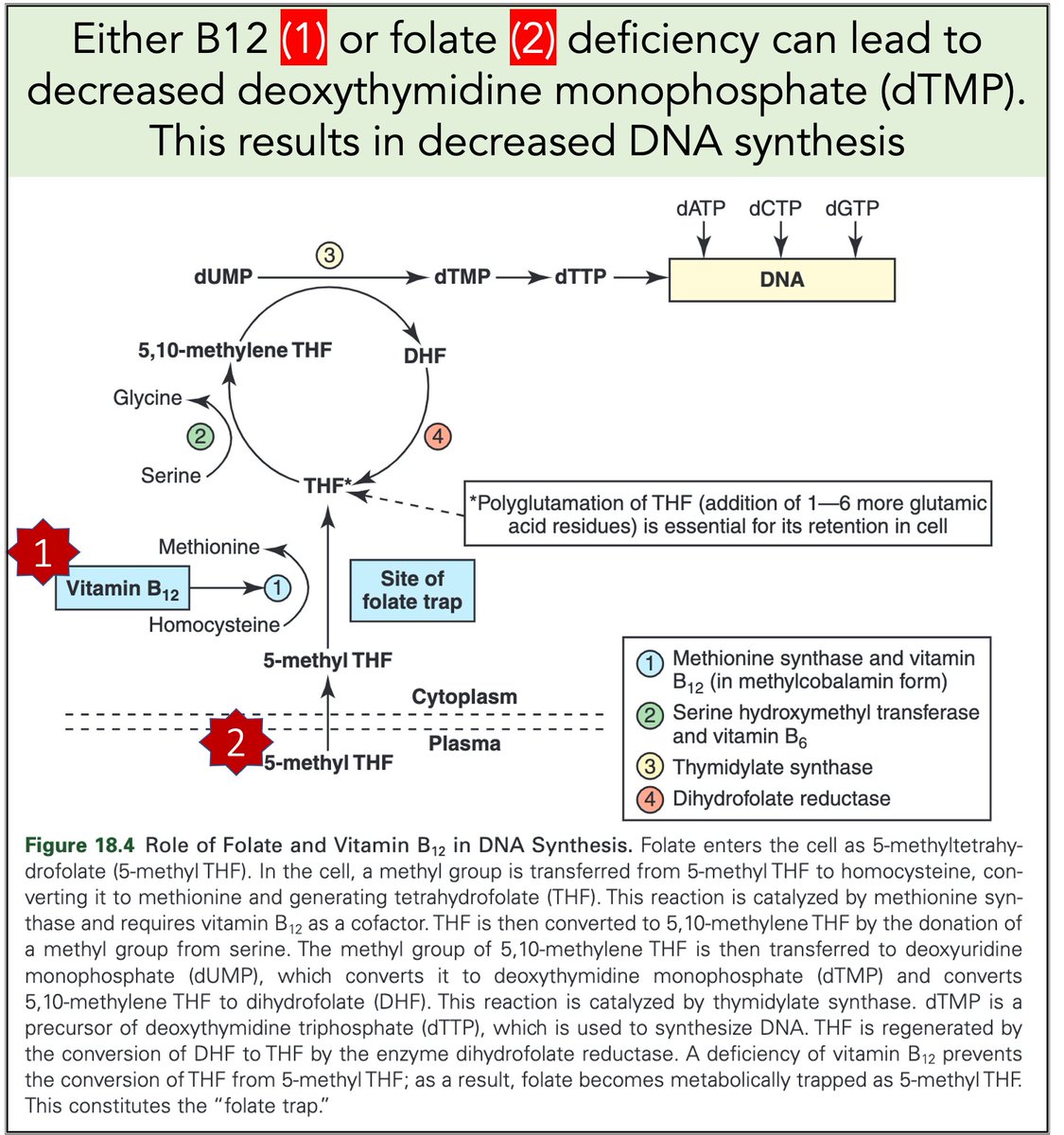
Why do B12 and folate deficiencies lead to HUGE red blood cells?
And, if the issue is DNA synthesis, why are red blood cells (which don't have DNA) the key cell line affected?
For answers, we'll have to go back a few billion years.

2/
RNA came first. Then, ~3-4 billion years ago, DNA emerged.
Among their differences:
🔹RNA contains uracil
🔹DNA contains thymine
But why does DNA contains thymine (T) instead of uracil (U)?
https://t.co/XlxT6cLLXg

3/
🔑Cytosine (C) can undergo spontaneous deamination to uracil (U).
In the RNA world, this meant that U could appear intensionally or unintentionally. This is clearly problematic. How can you repair RNA when you can't tell if something is an error?
https://t.co/bIZGviHBUc

4/
DNA's use of T instead of U means that spontaneous C → U deamination can be corrected without worry that an intentional U is being removed.
DNA requires greater stability than RNA so the transition to a thymine-based structure was beneficial.
https://t.co/bIZGviHBUc

5/
Let's return to megaloblastic anemia secondary to B12 or folate deficiency.
When either is severely deficient deoxythymidine monophosphate (dTMP*) production is hindered. With less dTMP, DNA synthesis is abnormal.
[*Note: thymine is the base in dTMP]
https://t.co/AnDUtKkbZh

Sarcomeres in cardiac myocytes (heart muscle cells) are mechanically coupled to focal adhesions through dorsal stress fiber-like structures. #cardiotwitter #CellBiology
1/13
A thread based on Figure 1
A mature adult cardiac myocyte is packed with sarcomeres, whose contractile forces are coupled to the extracellular environment. With sarcomeres so close to the plasma membrane, how can we study the nature of this coupling?
2/13
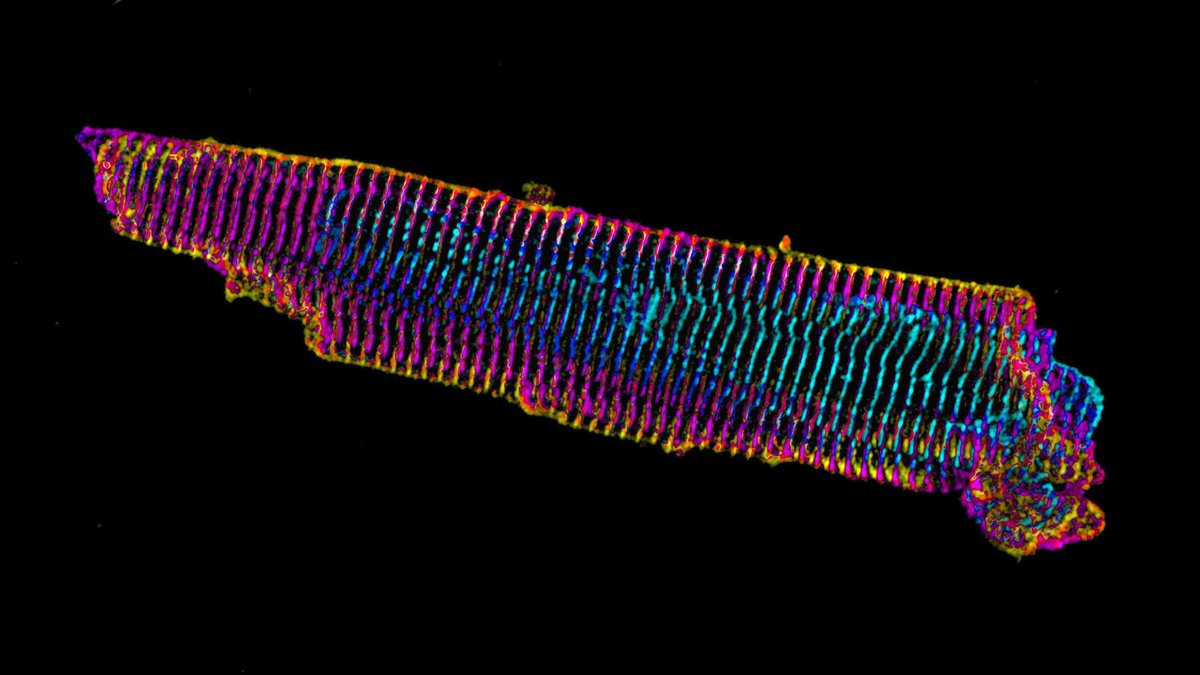
Short answer: find a model system where the sarcomeres are not so close to what the cardiac myocyte is attached to. Enter, iPS cell-derived cardiac myocytes. These are “immature” in culture as they resemble fetal or neonatal cardiac myocytes.
3/13
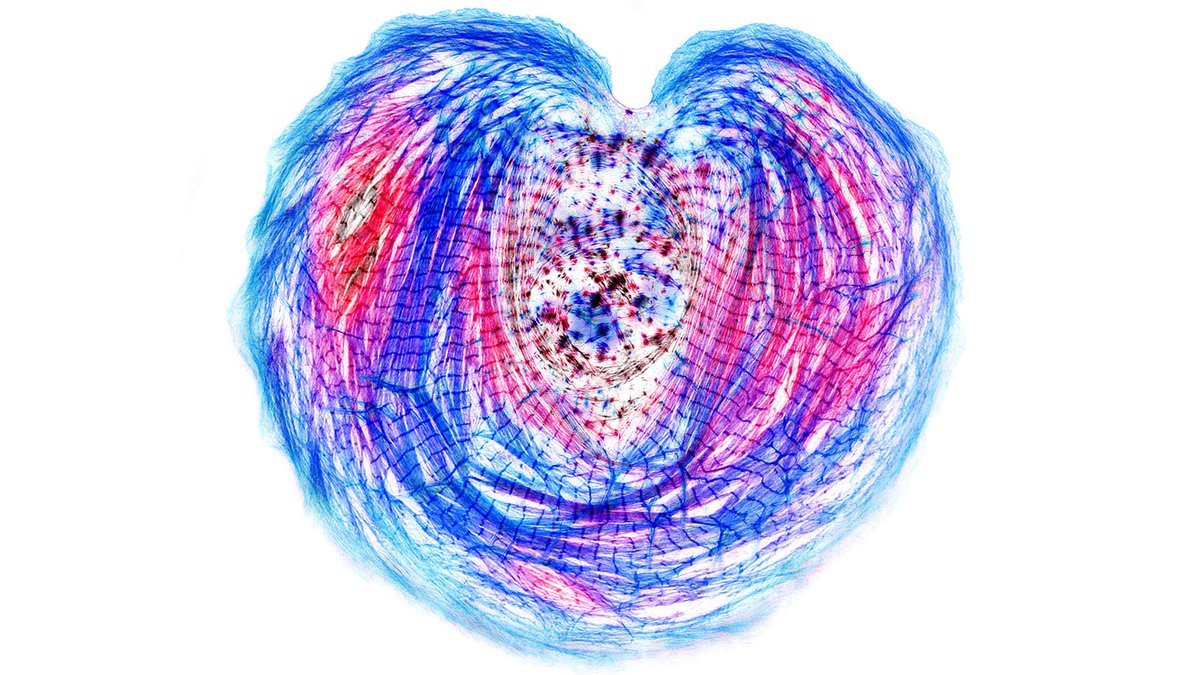
Our previous work on iPS cardiac myocytes reported that sarcomere containing myofibrils assembled on the top surface of the myocyte.
https://t.co/xIBCu3hG1W
4/13
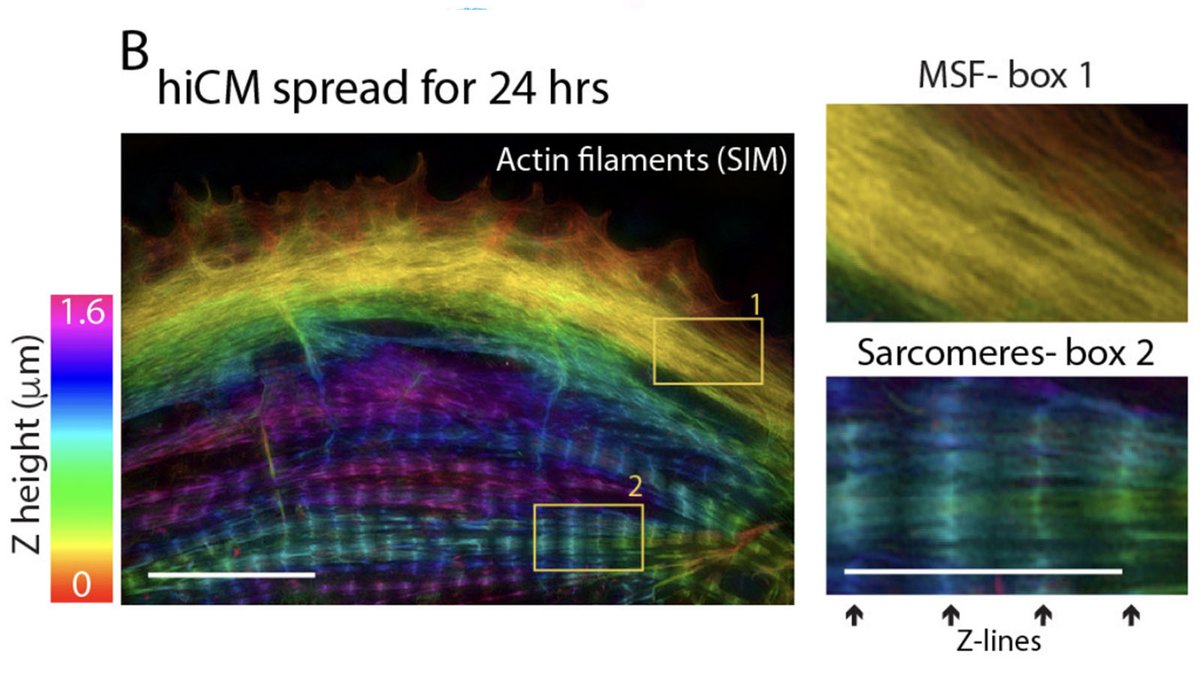
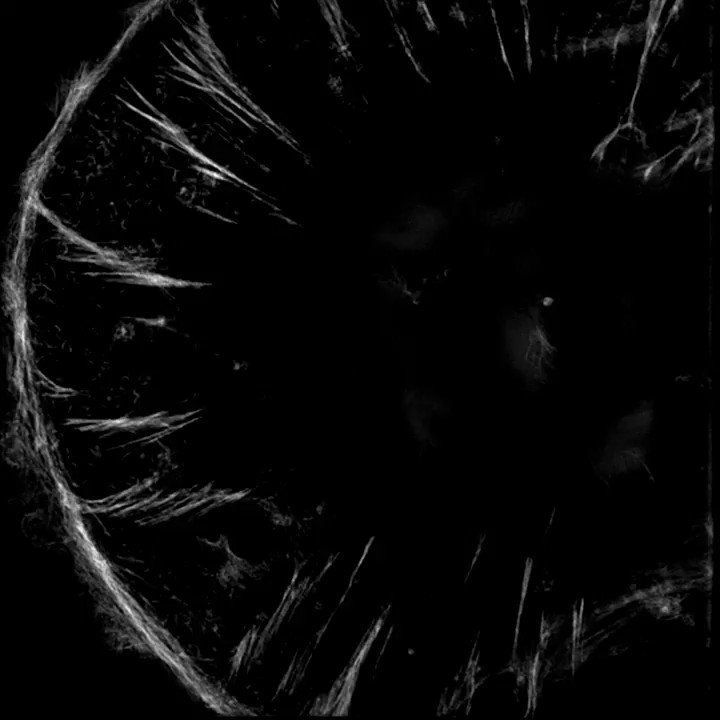
The sarcomeres seemed to be connected to focal adhesions on the bottom of the cell by thin actin bundles that resembled the dorsal stress fibers (DSF) commonly found in non-muscle cells. This movie steps through a Z stack of a myocyte starting at the bottom of the cell.
5/13
1/13
A thread based on Figure 1
A mature adult cardiac myocyte is packed with sarcomeres, whose contractile forces are coupled to the extracellular environment. With sarcomeres so close to the plasma membrane, how can we study the nature of this coupling?
2/13

Short answer: find a model system where the sarcomeres are not so close to what the cardiac myocyte is attached to. Enter, iPS cell-derived cardiac myocytes. These are “immature” in culture as they resemble fetal or neonatal cardiac myocytes.
3/13

Our previous work on iPS cardiac myocytes reported that sarcomere containing myofibrils assembled on the top surface of the myocyte.
https://t.co/xIBCu3hG1W
4/13

The sarcomeres seemed to be connected to focal adhesions on the bottom of the cell by thin actin bundles that resembled the dorsal stress fibers (DSF) commonly found in non-muscle cells. This movie steps through a Z stack of a myocyte starting at the bottom of the cell.
5/13

You May Also Like
https://t.co/6cRR2B3jBE
Viruses and other pathogens are often studied as stand-alone entities, despite that, in nature, they mostly live in multispecies associations called biofilms—both externally and within the host.
https://t.co/FBfXhUrH5d

Microorganisms in biofilms are enclosed by an extracellular matrix that confers protection and improves survival. Previous studies have shown that viruses can secondarily colonize preexisting biofilms, and viral biofilms have also been described.

...we raise the perspective that CoVs can persistently infect bats due to their association with biofilm structures. This phenomenon potentially provides an optimal environment for nonpathogenic & well-adapted viruses to interact with the host, as well as for viral recombination.

Biofilms can also enhance virion viability in extracellular environments, such as on fomites and in aquatic sediments, allowing viral persistence and dissemination.

Viruses and other pathogens are often studied as stand-alone entities, despite that, in nature, they mostly live in multispecies associations called biofilms—both externally and within the host.
https://t.co/FBfXhUrH5d

Microorganisms in biofilms are enclosed by an extracellular matrix that confers protection and improves survival. Previous studies have shown that viruses can secondarily colonize preexisting biofilms, and viral biofilms have also been described.

...we raise the perspective that CoVs can persistently infect bats due to their association with biofilm structures. This phenomenon potentially provides an optimal environment for nonpathogenic & well-adapted viruses to interact with the host, as well as for viral recombination.

Biofilms can also enhance virion viability in extracellular environments, such as on fomites and in aquatic sediments, allowing viral persistence and dissemination.