What are CROs, CDMOs, CMOs and CRAMS? How are they different from each other and what value do they add to the Pharma Value Chain? To understand these concepts, we took a look at the drug discovery process.
Thread on Drug Development process 🧵
Hit like & Retweet for more learnings ! 💊🧪🩺💉
@unseenvalue
Topics Covered :
1.Introduction to CRO,CDMO,CMO & CRAMS
2.Discovery
3.Clinical Trials
4.Commercialization and Surveillance
5.Success Rates
6.CRO
7.CMO
8.CDMO
9.CRAMS
What are CROs, CDMOs, CMOs and CRAMS? How are they different from each other and what value do they add to the Pharma Value Chain? To understand these concepts, we took a look at the drug discovery process.
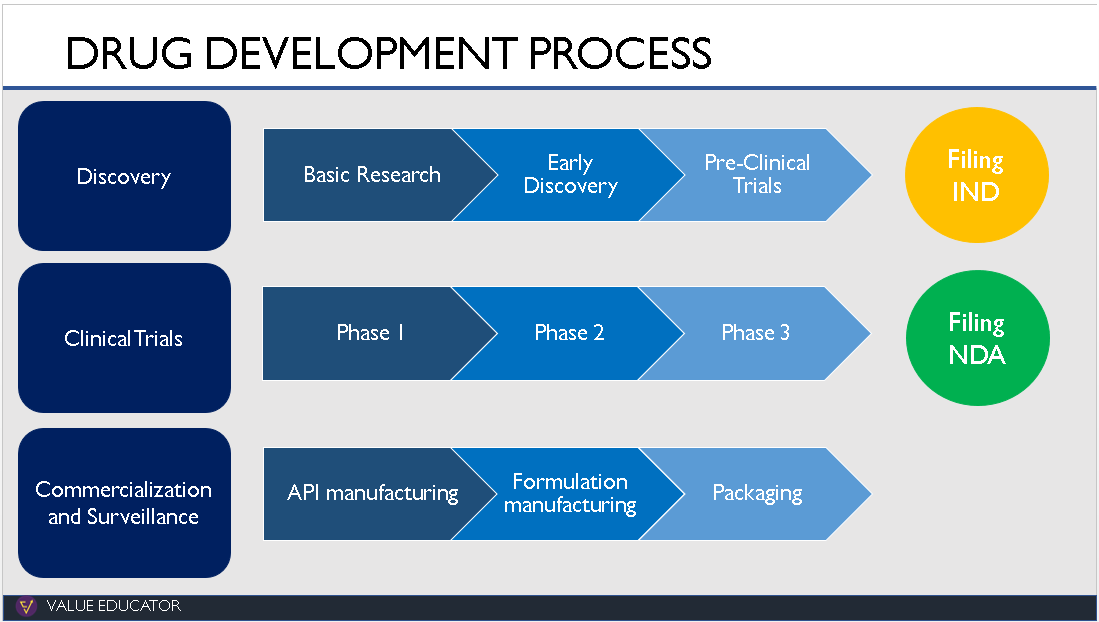
The discovery of a new drug starts in the research lab where researchers study hundreds of diseases. They analyse what part of the body a disease affects and what reaction the body has to these diseases.
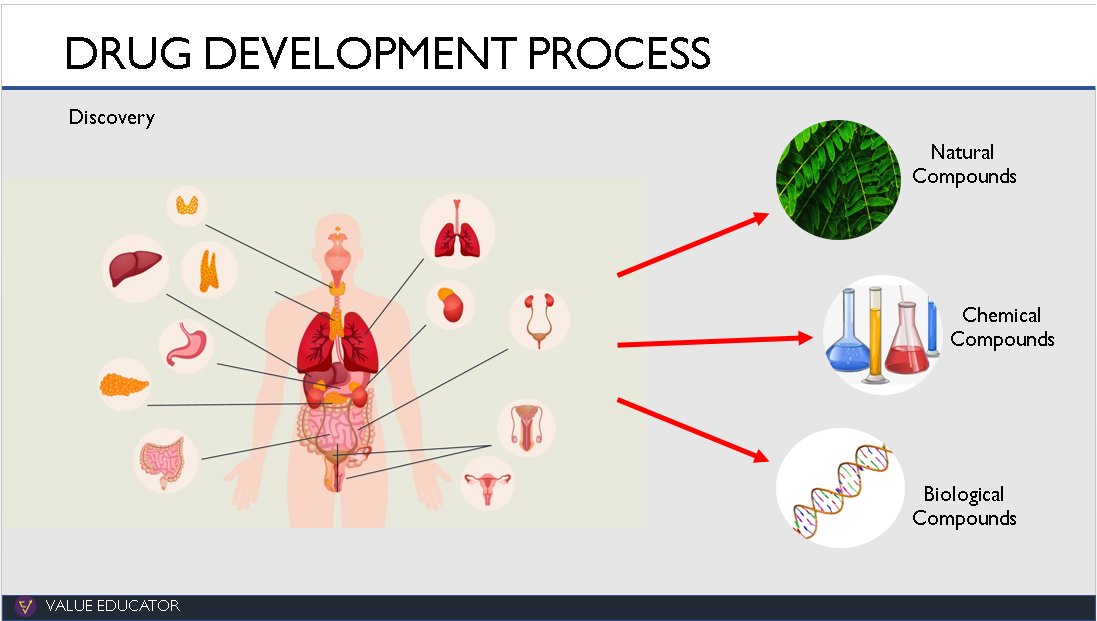
Once all this data has been collected, they file an Investigational New Drug (IND) Application with the US FDA. The US FDA reviews the IND within 6 months of filing. Once the IND is approved,
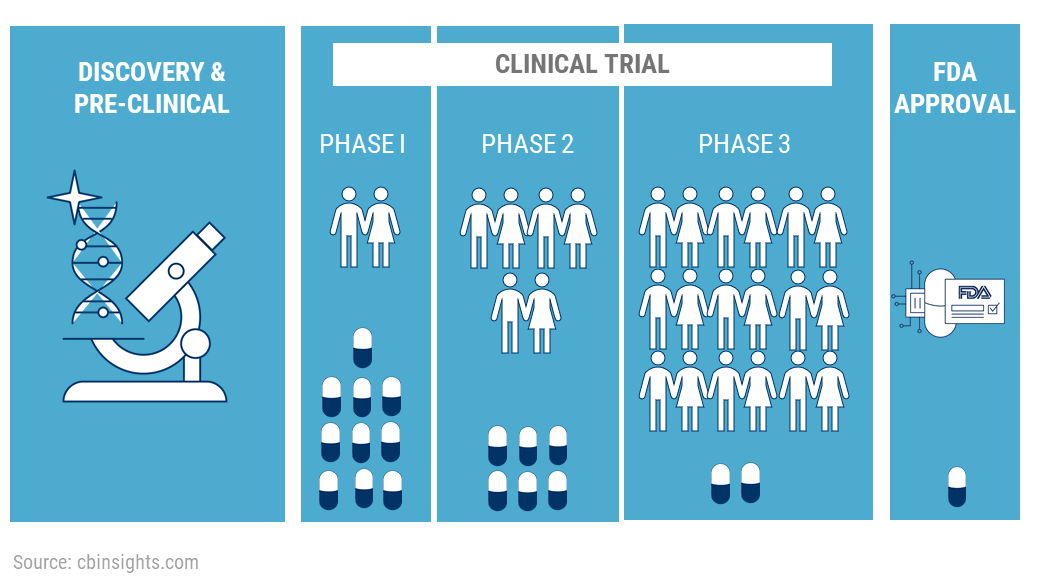
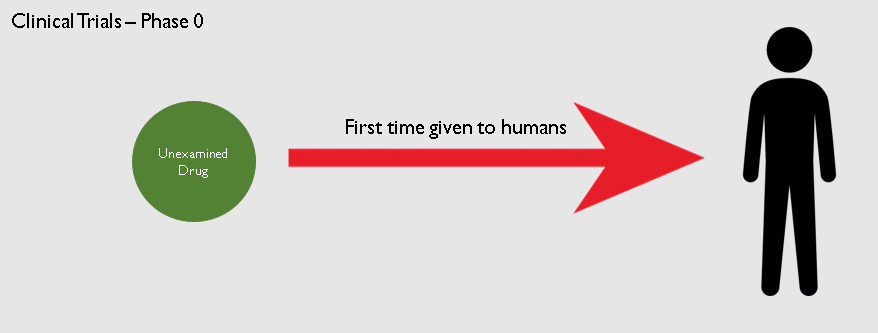
3. Clinical Trials:
The drug now moves to the Clinical Trials phase. It will be the first time a novel drug will be given to human beings. The clinical trials itself take place in 3 phases.
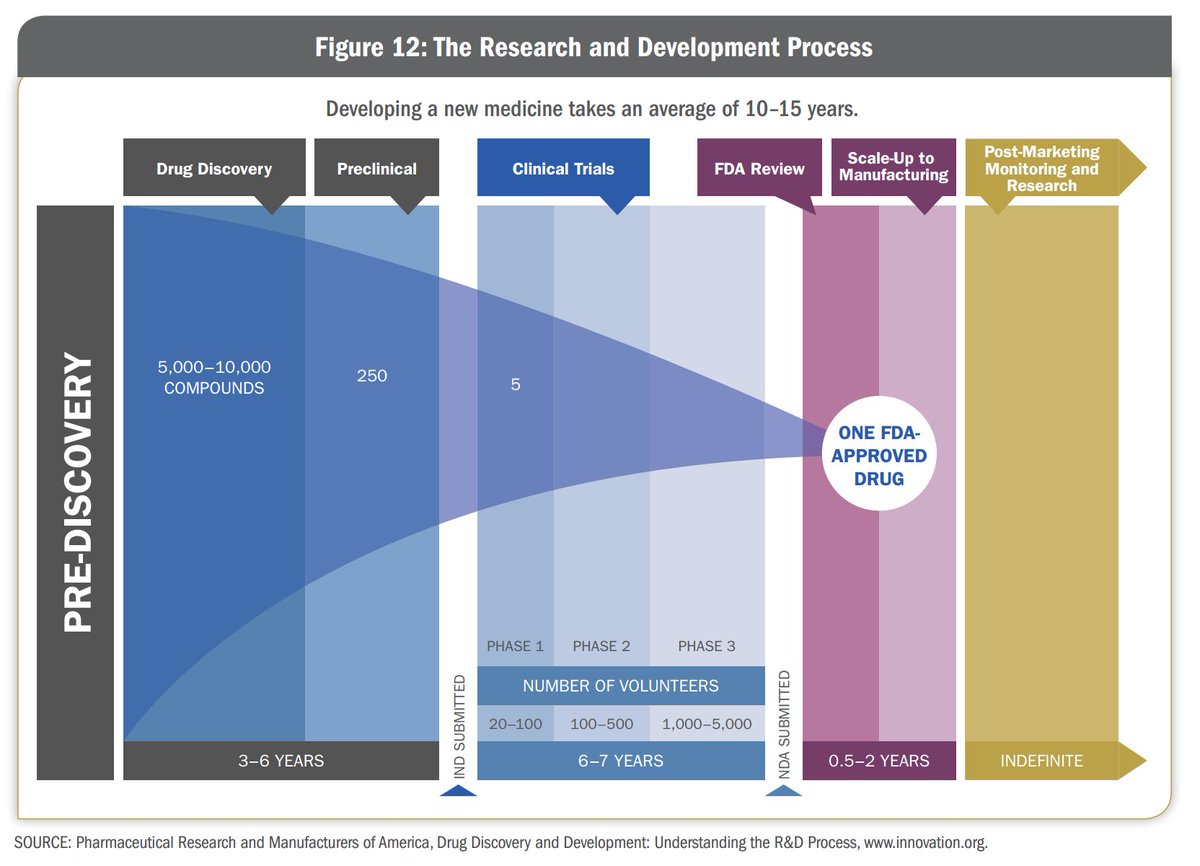
If the FDA approves the NDA, the company can manufacture and market the drugs to the public. But the testing does not end here. The drug now moves into Phase 4 testing.
Approximately 70% of the drugs move from Phase 1 to Phase 2, 33% move from Phase 2 to Phase 3, 25-30% move from Phase 3 to Phase 4. About 70-90% of drugs in Phase 4 are successful in staying in the market.
So where do the CROs and CDMOs come in? Contract research organizations help with the first 2 phases whereas Contract development and manufacturing organizations help with the last 2 phases
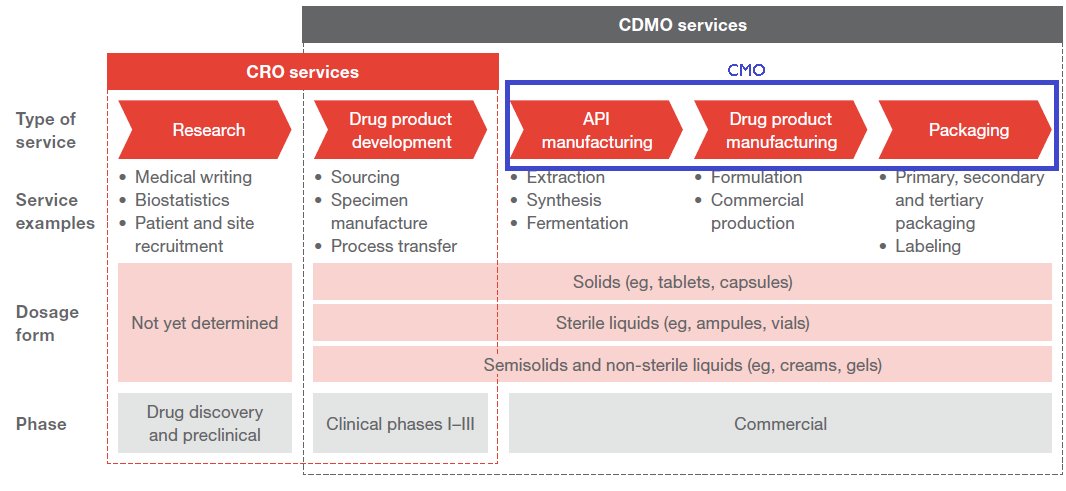
CROs support the innovators by providing services from drug discovery right up to commercialization. They provide services like target discovery, pre clinical trials, management of clinical trials, help with regulatory filings and pharmacovigilance(Phase 4).

Contract manufacturing organizations (CMO) on the other hand provide outsourced manufacturing solutions to the innovator. They are involved with the manufacturing of APIs and Formulations and oftentimes even involved with the final packaging of the product.
More from Value Educator
More from Uvlearnings
Conviction | Patience
Asian Paints is the TCS of 'Home Improvement' space
Think in terms of ::
- Sustainability of Leadership over decades
- Sustainability of Consumption Megatrend
- Sustainability of CFO and Governance
- Quality of Balance Sheet
Rather than absolute MCap
Asian Paints is the TCS of 'Home Improvement' space
Think in terms of ::
- Sustainability of Leadership over decades
- Sustainability of Consumption Megatrend
- Sustainability of CFO and Governance
- Quality of Balance Sheet
Rather than absolute MCap
Conviction | Patience
— Sajal Kapoor (@unseenvalue) December 30, 2019
TCS of CDMO in the making over the next 15Y \U0001f1ee\U0001f1f3
Compounding @ 25% since the IPO.
Syngene | Revisit 2030-2040 https://t.co/PHd8UEod5X
Research in CDMO/APIs is never a slam dunk. It may take ages to yield results. CEP filings can be lumpy as a result. As I wrote last year (link below), Hikal has been consistently sacrificing operating profits for a sustainable future. Best is ahead 🎥 🍿
https://t.co/hItmOud8Pm
https://t.co/hItmOud8Pm
#Hikal has stepped up R&D in recent years. Next few years (capex & launches) should yield superior earnings. Wish them all the luck \U0001f44d
— Sajal Kapoor (@unseenvalue) September 26, 2020
D: invested and biased view for sure, but it was below 1x price to sales vs 12x price to sales for many chemicals and generic players. No reco!! pic.twitter.com/aqPUKbziHK
You May Also Like
@franciscodeasis https://t.co/OuQaBRFPu7
Unfortunately the "This work includes the identification of viral sequences in bat samples, and has resulted in the isolation of three bat SARS-related coronaviruses that are now used as reagents to test therapeutics and vaccines." were BEFORE the

chimeric infectious clone grants were there.https://t.co/DAArwFkz6v is in 2017, Rs4231.
https://t.co/UgXygDjYbW is in 2016, RsSHC014 and RsWIV16.
https://t.co/krO69CsJ94 is in 2013, RsWIV1. notice that this is before the beginning of the project
starting in 2016. Also remember that they told about only 3 isolates/live viruses. RsSHC014 is a live infectious clone that is just as alive as those other "Isolates".
P.D. somehow is able to use funds that he have yet recieved yet, and send results and sequences from late 2019 back in time into 2015,2013 and 2016!
https://t.co/4wC7k1Lh54 Ref 3: Why ALL your pangolin samples were PCR negative? to avoid deep sequencing and accidentally reveal Paguma Larvata and Oryctolagus Cuniculus?
Unfortunately the "This work includes the identification of viral sequences in bat samples, and has resulted in the isolation of three bat SARS-related coronaviruses that are now used as reagents to test therapeutics and vaccines." were BEFORE the

chimeric infectious clone grants were there.https://t.co/DAArwFkz6v is in 2017, Rs4231.
https://t.co/UgXygDjYbW is in 2016, RsSHC014 and RsWIV16.
https://t.co/krO69CsJ94 is in 2013, RsWIV1. notice that this is before the beginning of the project
starting in 2016. Also remember that they told about only 3 isolates/live viruses. RsSHC014 is a live infectious clone that is just as alive as those other "Isolates".
P.D. somehow is able to use funds that he have yet recieved yet, and send results and sequences from late 2019 back in time into 2015,2013 and 2016!
https://t.co/4wC7k1Lh54 Ref 3: Why ALL your pangolin samples were PCR negative? to avoid deep sequencing and accidentally reveal Paguma Larvata and Oryctolagus Cuniculus?