This is a limited point about availability of efficacy data for vaccines under development in the context of the approval for CovidShield and Covaxin in India.
There have been many so-called experts on the idiotbox opining about apparent availability of P III data which 1/n
Here is one set of efficacy data post the interim analysis of a mRNA vaccine.
Source: https://t.co/BAPnP3PxEb
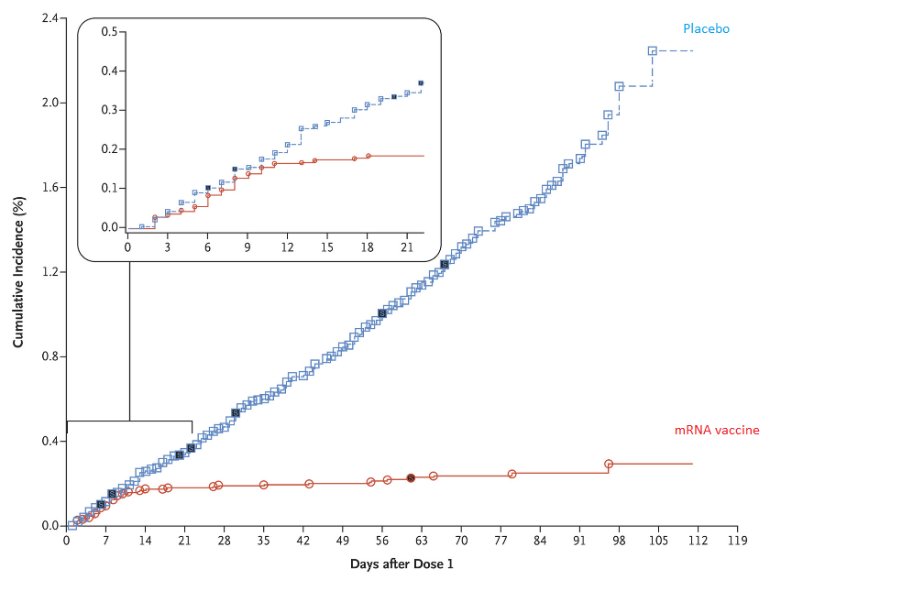
How does the SEC, or the sponsor of these studies, or the experts who are offering their opinion liberally on the idiotbox know what the efficacy is
A. Do they know if the blind was broken for the bridging study and the Phase III study?
B. If so, can they produce data like the one above showing how many subjects who were infected were
And if they cannot answer this question, then ask the following question:
C. In the absence of efficacy data, how does one claim that the vaccine candidate is effective?
D. Do they agree that therapeutic candidates ought to be approved
More from Health
🚨New lockdown regulations just published, in force tomorrow
The Health Protection (Coronavirus, Restrictions) (No. 3) and (All Tiers) (England) (Amendment) Regulations 2021
https://t.co/L5jwlTDaIE
(Thread)

These are not a new set of regulations: they are amendments an old set of regulations
Which we thought were gone! But they are back
Welcome back No.3 regulations
A quick thing before we continue!
I have been analysing these laws for free for 9 months now - if you want to say thanks and have a few £ to spare please give to my @LawCentres fundraiser
They give free legal advice to people who need it
They also amend the All Tiers regulations
Oh god it's all amendments by paragraph references
Basically all of England now in Tier 4 and Tier 4 is amended but not by a huge amount
This really is a terrible way to make laws on the fly - who can possibly understand it?!
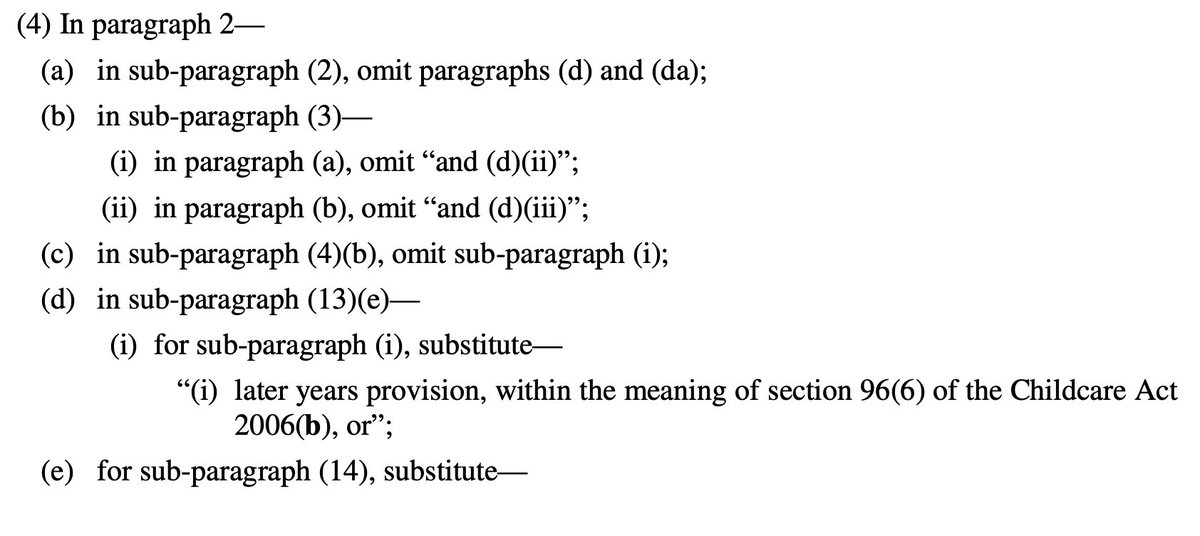
So, to explain, you need 2 documents open if you want to understand what is going on:
All Tiers regulations (Tiers 1-4, 2 December as amended) https://t.co/IraPQ112ak
And amendments https://t.co/L5jwlTDaIE
No sensible way of doing except by track changes, on it now, back soon
The Health Protection (Coronavirus, Restrictions) (No. 3) and (All Tiers) (England) (Amendment) Regulations 2021
https://t.co/L5jwlTDaIE
(Thread)

These are not a new set of regulations: they are amendments an old set of regulations
Which we thought were gone! But they are back
Welcome back No.3 regulations
A quick thing before we continue!
I have been analysing these laws for free for 9 months now - if you want to say thanks and have a few £ to spare please give to my @LawCentres fundraiser
They give free legal advice to people who need it
They also amend the All Tiers regulations
Oh god it's all amendments by paragraph references
Basically all of England now in Tier 4 and Tier 4 is amended but not by a huge amount
This really is a terrible way to make laws on the fly - who can possibly understand it?!

So, to explain, you need 2 documents open if you want to understand what is going on:
All Tiers regulations (Tiers 1-4, 2 December as amended) https://t.co/IraPQ112ak
And amendments https://t.co/L5jwlTDaIE
No sensible way of doing except by track changes, on it now, back soon
Let's talk honestly about "informed consent."
Someone with decades of training gives someone with none advice usually packed into 1-3 mins. Huge amount is based on trust. Huge potential for bias built in. But also there is no obligation to provide real alternative options.
I am classified as 'gifted' (obnoxious and ableist term). I mention because of what I am about to say. You all know that I was an ambulatory wheelchair user previously - could stand - but contractures have ended that. When I pleaded for physio, turned down. But did you know...
I recently was chatting with a doctor I know and explaining what happened and the day the physiatrist told me it was too late and nothing could be done. The doctor asked if I'd like one of her friends/colleagues to give second opinion. I said yes please! So...
She said can you send me MRI and other imaging they did to determine it wasn't possible to address your contractures.
Me: What?
Dr.: They did a MRI first before deciding right?
Me: No
Dr: What did they do??!
Me: Examined me for 2 minutes.
Dr: I am very angry rn. Can't talk.
My point is you don't even know if you are making "informed" decisions because the only source of information you have is the person who has already decided what they think you should do. And may I remind you of a word called 'compliance.'
Someone with decades of training gives someone with none advice usually packed into 1-3 mins. Huge amount is based on trust. Huge potential for bias built in. But also there is no obligation to provide real alternative options.
MAiD isn't eugenics. The task for the medical profession is to ensure informed consent. Failures on that front should result in enforcement of the law. But Bill C-7 is the result of the existing regime imposing unnecessary, unconstitutional harms by blocked access to MAiD.
— Emmett Macfarlane (@EmmMacfarlane) February 13, 2021
I am classified as 'gifted' (obnoxious and ableist term). I mention because of what I am about to say. You all know that I was an ambulatory wheelchair user previously - could stand - but contractures have ended that. When I pleaded for physio, turned down. But did you know...
I recently was chatting with a doctor I know and explaining what happened and the day the physiatrist told me it was too late and nothing could be done. The doctor asked if I'd like one of her friends/colleagues to give second opinion. I said yes please! So...
She said can you send me MRI and other imaging they did to determine it wasn't possible to address your contractures.
Me: What?
Dr.: They did a MRI first before deciding right?
Me: No
Dr: What did they do??!
Me: Examined me for 2 minutes.
Dr: I am very angry rn. Can't talk.
My point is you don't even know if you are making "informed" decisions because the only source of information you have is the person who has already decided what they think you should do. And may I remind you of a word called 'compliance.'